Введение
Противоопухолевая цитостатическая терапия обычно оказывает повреждающее действие на различные органы и системы организма. Гепатотоксичность является одним из наиболее частых побочных эффектов химиотерапии и зависит от препарата, доз и схем его применения, а также сопутствующих хронических заболеваний печени [1]. Печеночная токсичность может требовать изменения режима лечения, удлинения интервалов между курсами, а в ряде случаев – прекращения лекарственной терапии. Все это негативно сказывается на психическом состоянии пациентов и результатах лечения.
На сегодняшний день механизмы токсического повреждения печени цитостатиками до конца не изучены, а в современной отечественной литературе обнаруживается не так много работ, посвященных этой проблеме.
Эффективность гепатопротекторов связана с их правильным и своевременным назначением, при том что реальная практика их применения зачастую является хаотичной и неадекватной.
Метаболизм лекарственных препаратов в печени проходит несколько основных этапов: первый – с участием системы цитохрома Р450, монооксигеназ и других ферментов; второй – биотрансформация, конъюгация метаболитов с эндогенными молекулами; третий – транспорт и экскреция продуктов биотрансформации. Важную роль в развитии токсического повреждения печени играют реактивные метаболиты и свободные радикалы. Реактивные метаболиты связываются с белками и липидами мембран, вызывая их перекисное окисление [2]. Это ведет к развитию структурных и функциональных нарушений, повышающих активность аминотрансфераз и вызывающих гибель гепатоцитов [3].
Цитостатики могут вызывать любые известные повреждения печени, включая некроз, стеатоз, фиброз, холестаз и поражение сосудов [4, 5]. До 90 % проявлений гепатотоксичности включают:
1) острый гепатоцеллюлярный гепатит,
2) острый холестатический гепатит и
3) смешанный гепатит [6].
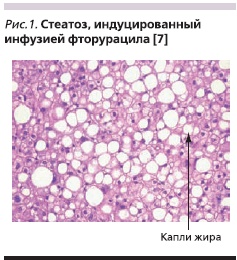
В 1920 г. впервые описана обструкция и дилатация печеночных синусов (венооклюзионное заболевание) как летальная интоксикация в результате употребления ядовитых растений. Данная морфологическая форма характеризуется повреждением синусоидальных эндотелиальных клеток вследствие активации металлопротеиназ и оксидативного стресса [7].На рис. 1 представлена морфологическая картина индуцированного цитостатиками стеатоза, которая характеризуется наличием больших капель жира в гепатоцитах. В среднем частота стеатоза составляет 30 %.
Стеатогепатит связан с оксидативным стрессом, следствием которого является повреждение митохондрий гепатоцитов.
Ниже представлены группы лекарственных препаратов, вызывающих различные варианты токсического повреждения печени [8]:
- алкилирующие агенты (циклофосфан, иофосфамид, мелфалан, хлорамбуцил и др.) могут приводить к развитию центролобулярного или перипортального повреждения, холестаза, в ряде случаев на фоне холестаза развивается воспаление;
- антиметаболиты (фторурацил – ФУ, меркаптопурин, метотрексат, гемцитабин) вызывают развитие венооклюзионной болезни, холестаз и оказывают прямое цитотоксическое повреждающее действие на гепатоциты;
- производные нитрозомочевины (кармустин, ломустин) приводят к истощению внутрипеченочных запасов глутатиона, что увеличивает риск окислительного повреждения печени;
- противоопухолевые антибиотики (доксорубицин, блеомицин, митомицин, дактиномицин, митоксантрон) повреждают мембрану гепатоцита с образованием свободных радикалов;
- винкаалколоиды и таксаны вызывают различные токсические повреждения печени, включая стеатогепатиты;
- ингибиторы топоизомеразы I (этопозид, иринотекан, топотекан) при биотрансформации образуют токсический метаболит SN-38;
- производные платины вызывают стеатозы, стеатогепатиты, венообструктивную болезнь;
- интерфероны, интерлейкины приводят к активации T-киллеров и цитокинов, а также к прямому токсическому повреждению гепатоцитов;
- гормонотерапия (тамоксифен, антиандрогены) может приводить к развитию холестаза;
- таргетная терапия (бевацизумаб) в ряде случаев ассоциируется с синусоидальной дилатацией.
Таким образом, в большей или меньшей степени, но каждая группа препаратов, используемых в современном лекарственном лечении злокачественных опухолей, оказывает воздействие на функциональное состояние печени.
Для оценки тяжести гепатотоксичности используются следующие клинико-лабораторные показатели (табл. 1) [8].
Таблица 1. Клинико-лабораторные показатели оценки тяжести гепатотоксичности.
Для диагностики токсического повреждения печени дополнительно можно использовать инструментальные методы (компьютерную, магнитно-резонансную томографии и др.), биопсию. По соотношению показателей АСТ/АЛТ можно судить о характере гиперферментемии [8]. В норме отношение АСТ к АЛТ ближе к 1,0. При отношении АСТ к АЛТ меньше 0,7 подтверждается печеночный характер гиперферментемии. Если отношение АСТ к АЛТ более 1,3, имеет место гиперферментемия внепеченочного генеза.
Тактика коррекции доз при гепатотоксичности в основном ориентирована на уровень повышения уровней общего билирубина и трансаминаз [8].
- При повышении уровня общего билирубина в 1,2–2,5, трансаминаз – в 2–5 раз дозы антрациклинов уменьшают на 50 %, а других цитостатиков – на 25 %.
- При повышении уровня общего билирубина в 2,6–5,0, трансаминаз – в 5–10 раз дозы антрациклинов уменьшают на 75 %, а других цитостатиков – на 50 %.
- Если отмечается дальнейшее увеличение лабораторных показателей, противоопухолевая терапия прекращается.
Специфическим антидотом при дозозависимой гепатотоксичности являются N-ацетилцистеин и другие доноры сульфгидрильной группы [9]. Лекарственная гепатотоксичность чаще всего ведет к прекращению противоопухолевого лечения или отсрочке применения гепатотоксичного препарата, иногда назначают симптоматическое лечение.
Эффективность кортикостероидов при иммуноаллергическом гепатите и урсодеоксихолевой кислоты при холангите убедительно не доказана [10, 11].
Hirata и соавт. продемонстрировали важность метилирования для обеспечения функции и целостности мембран гепатоцитов [12]. Глутатион выполняет ряд важных функций, включая нейтрализацию свободных кислородных радикалов, обмен тиосульфида, хранение и перенос цистеина, конъюгацию и нейтрализацию реактивных метаболитов при биотрансформации ксенобиотиков [13]. Недостаточность глутатиона приводит к повышению восприимчивости к окислительному стрессу. В печеночных клетках недостаток его также вызывает инактивацию адеметионинсинтетазы, что служит причиной нарастания истощения запасов глутатиона в печени [14].
Адеметионин (Гептрал ®) – основной эндогенный донор метильной группы в биологических реакциях трансметилирования [15]. Он необходим для синтеза нуклеиновых кислот и белка, играет основную роль в синтезе полиаминов и является источником цистеина, необходимого для образования глутатиона – важнейшего эндогенного гепатопротектора [15].
В экспериментальных исследованиях продемонстрирована эффективность адеметионина в лечении поражений печени, вызванных четыреххлористым углеродом, D-галактозамином, ацетаминофеном, алкоголем и др. [16–20]. В клинических исследованиях применение адеметионина позволило отложить трансплантацию печени и увеличить выживаемость у больных с алкогольным поражением этого органа [21]. Кроме того, препарат обеспечивает благоприятный эффект при внутрипеченочном холестазе, развивающемся у беременных, и хроническом неалкогольном поражении печени.
Целью настоящего исследования была оценка эффективности препарата Гептрал ® (адеметионин, соль SD-4) при лечении гепатотоксичности, индуцированной химиотерапией.
Мы использовали Гептрал ® (адеметионин, соль SD-4) в таблетках по 400 мг (фармацевтическая компания Эбботт).
Материал и методы
В исследование были включены 19 пациентов с различными злокачественными опухолями: колоректальный рак – 12, рак молочной железы – 3, рак желудка – 2, рак яичников – 1, рак правого надпочечника – 1.
Возраст больных составлял от 41 до 80 лет: 41–60 лет – 14 (73 %), 61–80 – 7 (27 %) человек. Общее состояние оценивалось по шкале ECOG: 1 – 10 пациентов, 2 – 9 больных. Несколько линий предшествующей химиотерапии до включения в настоящее исследование получали большинство пациентов (14/19–73,6 %).
Метастазы в печень исходно обнаружились у 7 из 19 (36,8 %) пациентов. Незначительное повышение уровня общего билирубина (от 21 до 22 мкмоль/л) выявлено у 4 больных с метастазами в печень, но не требовало выполнения дренирующих хирургических пособий. Уровень общего белка у всех пациентов был в пределах нормы (от 60 до 80 г/л).
Проводились следующие режимы химиотерапии:
- FAC: доксорубицин 50 мг/м2, ФУ 500 мг/м2, циклофосфан 500 мг/м2 1 раз в 3 недели;
- митомицин 6 мг/м2 + ралтитрексид 3 мг/м2 1 раз в 3 недели; • паклитаксел 135 мг/м2 + карбоплатин AUC-5 1 раз в 3 недели;
- блеомицин 10 мг/м2 (1–3-й день) + ФУ 500 мг/м2 (1–3-й день) + лейковорин 30 мг/м2 (1–3-й день), интервал – 3 недели;
- FOLFIRI – иринотекан 180 мг/м2, лейковорин 200 мг/м2 1–2-й день, ФУ – 400 мг/м2 1–2-й день, ФУ – 1200 мг/м2 48-часовая инфузия, интервал – 14 дней.
Адьювантное лечение получили 3 пациентки с раком молочной железы после радикальных операций, остальные 16 больных имели диссеминированный процесс и получали лечебную химиотерапию.
Критерием гепатотоксичности явилось увеличение активности АСТ и/или АЛТ в крови на фоне химиотерапии. Степень гепатотоксичности определена по наибольшему уровню одной из них. Уровень щелочной фосфатазы и других печеночных ферментов лабораторно рутинно не определялся и нами не оценивался. Активность ферментов определяли в одной лаборатории. В исследовании не было контрольной группы.
Гептрал ® при наличии гепатотоксичности назначался внутрь по 400 мг 2 раза в день ежедневно в течение минимум 4 недель на фоне химиотерапии. Оценка динамики уровня трансаминаз проведена через 2, 3, 4 и более недель от начала применения Гептрала ®.
Распределение больных по степени гепатотоксичности на момент включения в исследование представлено в табл. 2. Первая степень имела место у 12 больных, 2-я – у 6, 3-я – у 1. Из 7 больных с метастазами в печень 1-я степень гепатотоксичности отмечена у 2 пациентов, 2-я – у 4. У одной больной зарегистрирована остро развившаяся 3-я степень гепатотоксичности, что потребовало прекращения химиотерапии.
Таблица 2. Распределение больных по уровню трансаминаз.
Результаты
Как указывалось выше, эффективность терапии препаратом Гептрал ® анализировали на основании динамики активности трансаминаз через 2, 3, 4 и более недель после начала лечения. Статистическая обработка данных не применялась в связи с небольшим количеством наблюдений. Уровень билирубина нами контролировался, но не оценивался, т. к. не являлся критерием оценки и оставался незначительно повышенным у 4 пациентов с метастазами в печень на фоне положительной динамики трансаминаз. Напомним, что больные с гепатотоксичностью 1-й и 2-й степенями получали Гептрал на фоне химиотерапии.
Уровень АСТ и АЛТ через 2 недели лечения существенно не изменился ни у одного пациента (рис. 2).
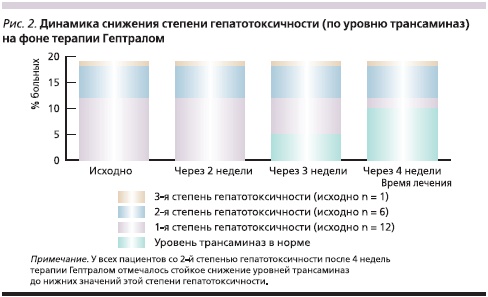
Через 3 недели в группе пациентов с 1-й степенью гепатотоксичности уровень АСТ снизился до нормы (менее 37 ЕД/л) у 3 человек, АЛТ (менее 40 ЕД/л) – у 2 пациентов. В группе больных со 2-й и 3-й степенями гепатотоксичности через 3 недели лечения уровень трансаминаз оставался стабильным.
Через 4 недели печеночные показатели снизились до нормы еще у 5 пациентов с 1-й степенью гепатотоксичности. Кроме того, отмечено снижение показателей АСТ и АЛТ у всех пациентов со 2-й степенью гепатотоксичности, в среднем до 100 ЕД/л, т. е. до нижней границы значений, характеризующих эту степень поражения печени.
Таким образом, через 4 недели уровень трансаминаз снизился до нормы у 10 из 12 пациентов с 1-й степенью гепатотоксичности, т. е. эффективность Гептрала ® составила 83,3 %. При 2-й степени гепатотоксичности показатели трансаминаз также снизились, но не достигли уровня, характерного для 1-й степени гепатотоксичности.
Продолжение приема Гептрала ® еще в течение 2 недель (до 6 недель) позволило добиться нормализации уровня трансаминаз у остальных двух пациентов с 1-й степенью гепатотоксичности.
Для нормализации уровня трансаминаз при 2-й степени гепатотоксичности потребовался более длительный прием Гептрала ®: у 2 пациентов – 2 месяца, у 1 – 3, у 3 – 4 месяца (химиотерапия продолжалась без изменения доз).
У пациентки с 3-й степенью гепатотоксичности, имевшей метастазы в печень, уровень трансаминаз снизился до 1-й степени (89 ЕД/л) после 4 месяцев приема Гептрала ®.
Прием Гептрала ® в режиме по 400 мг внутрь 2 раза в день в течении всего срока терапии не вызывал каких-либо побочных реакций у пациентов.
Заключение
Проведенное исследование показало эффективность Гептрала ® при лечении гепатотоксичности, обусловленной химиотерапией:
- Гептрал, назначаемый в дозировке 400 мг 2 раза в сутки в течение 4 недель пациентам с 1-й степенью гепатотоксичности на фоне полихимиотерапии (ПХТ), позволяет полностью устранить проявления синдрома цитолиза у 83,3 % пациентов без изменения схем ПХТ. Продление курса терапии еще на 2 недели обеспечивает нормализацию сывороточных трансаминаз у всех больных данной группы.
- Гептрал в указанном режиме применения стабилизирует уровни АСТ и АЛТ у больных со 2-й степенью гепатотоксичности, удерживая уровень трансаминаз на нижней границе, характерной для этой степени поражения печени. Это позволяет пациентам получать ПХТ в полном объеме и в запланированные сроки.
- Для нормализации сывороточных трансаминаз у пациентов со 2-й степенью гепатотоксичности требуется продление курса терапии Гептралом ® до 2–4 месяцев без отклонений от режима ПХТ.
- При приеме внутрь по 400 мг 2 раза в день Гептрал ® не вызывает побочных реакций и хорошо переносится больными. Таким образом, Гептрал ® может быть рекомендован в рамках сопроводительной терапии для лечения гепатотоксичности, возникшей в процессе цитостатической химиотерапии.
Информация об авторах:
Снеговой Антон Владимирович – кандидат медицинских наук, старший научный сотрудник,
отделение изучения новых противоопухолевых лекарств, РОНЦ им. Н.Н.Блохина.
Тел. 8 (495) 324-41-09
Манзюк Людмила Валентиновна – доктор медицинских наук, профессор кафедры онкологии
ФПДО МГМСУ, заведующая отделением изучения новых противоопухолевых лекарств, РОНЦ им. Н.Н. Блохина.
Тел. 8 (495) 324-25-74


