Staphylococcus aureus – грамположительный микроорганизм, вызывающий инвазивные инфекции различной локализации, которые обусловливают значительную заболеваемость и смертность населения [1]. Он был первым микроорганизмом, у которого была обнаружена устойчивость к антибиотикам вскоре после введения пенициллинов в медицинскую практику (табл. 1). В настоящее время особую проблему представляет метициллинорезистентный S. aureus (MRSA – Methicillin-Resistant S. aureus), впервые выявленный в начале 1960-х гг. [2, 3]. Во всем мире он является самым распространенным грамположительным возбудителем нозокомиальных инфекций различной локализации [2, 3]. Распространенность MRSA составляет в среднем по Европе 20%, достигая в отдельных странах 50, в США – 33–60% [4–7]. По данным Европейского центра по контролю над инфекциями (ЕСDС – European Centre for Disease Prevention and Control), на континенте ежегодно регистрируется 170 тыс. случаев инфекции, вызванной MRSA, в т.ч. 5000 случаев с летальным исходом [8]. MRSA является причиной более 1 млн дополнительных койко-дней в год, экономические затраты европейской системы здравоохранения, связанные с инфекциями, вызванными данным возбудителем, оцениваются примерно в 380 млн евро [8]. В США MRSA вызывает более 60% инфекций у пациентов, госпитализированных в отделения интенсивной терапии, и 18 650 летальных исходов в год среди госпитализированных больных [9–11]. Ежегодное число смертей госпитальных больных от инфекций, вызванных MRSA, аналогично общему числу смертей, связанных со СПИДом, туберкулезом и вирусным гепатитом [12]. К группам риска развития этих инфекций относятся пациенты с установленными катетерами и/или подвергшиеся хирургическим вмешательствам, больные бронхолегочными заболеваниями, сахарным диабетом, новорожденные, пожилые и лица с иммунодефицитом.
Начиная с 2000-х гг. инфекции, вызванные MRSA, перестали быть исключительно внутрибольничной проблемой в связи с широким распространением в ряде стран, прежде всего в Северной Америки, внебольничного MRSA (CA-MRSA – CommunityAcquired Methicillin-Resistant S. aureus), который в отличие от госпитальных штаммов вызывает инфекции у негоспитализированных лиц, часто не имеющих типичных факторов риска, таких как сопутствующие заболевания и внутривенная наркомания [14–16]. В США и Канаде CA-MRSA в настоящее время является одним из основных возбудителей инфекций кожи и мягких тканей, включая абсцессы, целлюлит и некротизирующий фасциит [14, 15], а также вызывает серьезные инфекции другой локализации – тяжелый многоочаговый остеомиелит, бактериемию, септический шок и некротизирующую пневмонию [16–18]. CA-MRSA может быть и этиологическим фактором нозокомиальных инфекций [19, 20].
CA-MRSA передается преимущественно от человека к человеку, однако может также передаваться человеку от домашних и, возможно, диких животных, в частности грызунов [21–24]. Стафилококк зоонозного происхождения (LA-MRSA) широко распространен среди коров, домашних свиней, коз, лошадей [25, 26]. Его также выделяют у домашней птицы, собак, кошек и других домашних питомцев.

MRSA имеет экстраординарный спектр вирулентности, связанный с образованием пирогенных токсинов суперантигенов, адгезинов и деструктивных ферментов, что обусловливает широкий спектр заболеваний, вызываемых этим возбудителем [13]. Вопрос о более высокой вирулентности MRSA по сравнению с метициллиночувствительными штаммами S. aureus (MSSA – Methicillin-Sensitive S. aureus) продолжает дискутироваться [27]. В некоторых исследованиях выявлена более высокая заболеваемость и смертность при инфекциях, вызванных MRSA. Например, септицемия, обусловленная этим возбудителем, ассоциировалась с двукратным повышением частоты летальных исходов по сравнению с септицемией, вызванной MSSA [28, 29]. В других исследованиях не было показано повышения летальности среди пациентов с бактериемией и вентилятороассоциированной пневмонией, вызванной MSSA [30, 31]. Однако существует четкая ассоциация инфекций, вызванных MRSA, с более высокой стоимостью лечения, которая в 2 раза превышает таковую для инфекций, вызванных MSSA [29, 32]. Повышение стоимости лечения преимущественно связано с более высокой стоимостью госпитализации, что в значительной степени обусловлено ограниченным выбором антибиотиков, эффективных в отношении данного возбудителя [30]. Нозокомиальные штаммы MRSA помимо β-лактамов проявляют резистентность и к антибиотикам других классов, причем устойчивость S. aureus ко многим из них развивается достаточно быстро (табл. 1).
CA-MRSA в отличие от нозокомиальных штаммов пока сохраняет высокую чувствительность к «небеталактамным» антибиотикам, в т.ч. к гентамицину, ко-тримоксазолу, линезолиду, хинупристин/дальфопристину и хлорамфениколу [33].
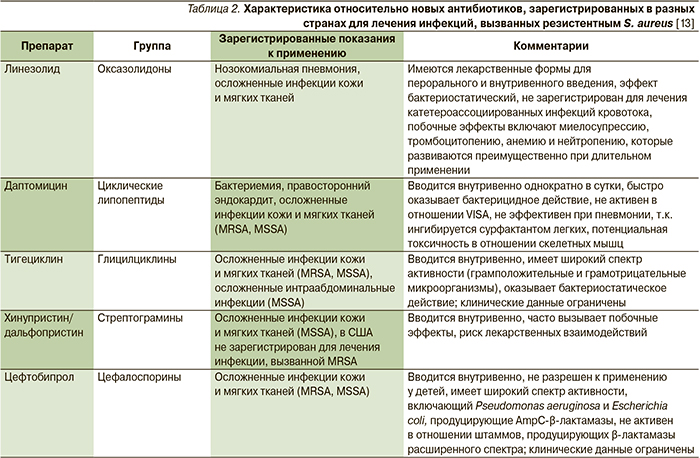
Основными препаратами лечения инфекций, вызванных MRSA, за последние два десятилетия остаются гликопептиды ванкомицин, тейкопланин ванкомицин и тейкопланин. С начала 1990-х гг. на мировой фармацевтический рынок поступило лишь несколько новых препаратов, зарегистрированных по данному показанию (табл. 2), что связано с постоянно сокращающимися в последние годы расходами ведущих фармкомпаний на разработку новых антибиотиков [34].
Между тем разработка новых антибиотиков, активных в отношении MRSA, остается крайне актуальной задачей, т.к. уже в 1997 г. из клинического материала был выделен штамм данного микроорганизма с умеренным уровнем резистентности (минимальная подавляющая концентрация – МПК=8 мг/л) к ванкомицину (VISA), а в настоящее время описаны единичные случаи выделения штаммов и с высоким уровнем резистентности к ванкомицину (VRSA) [35, 36]. При этом, несмотря на отсутствие истинной резистентности к ванкомицину у VISA с фармакологической точки зрения, эти штаммы резистентны к гликопептиду с клинической и биологической точек зрения, т.е. лечение ванкомицином заканчивается неудачей [37]. Например, в нескольких мета-анализах показано, что исходы лечения ванкомицином при широко распространенных инфекциях кожи и мягких тканей значительно ухудшаются в случаях, когда его МПК приближаются к 2 мг/л [38].
В мае 2014 г. для лечения инфекций кожи и мягких тканей, вызванных грамположительными микроорганизмами, включая MRSA, в США был разрешен новый антибиотик – далбаванцин [39]. Он был одобрен FDA для медицинского применения под торговым названием «Далванс» (Durata Therapeutics). Следует отметить, что производитель препарата (в то время Pfizer) уже получал одобрительное письмо на его использование от FDA (Food and Drug Administration) в декабре 2007 г. [40], однако в сентябре 2008-го добровольно отозвал препарат в связи с решением о проведении дополнительного клинического исследования [41].
На этот раз далбаванцин стал одним из первых антибиотиков, одобренных в рамках инициативы GAIN (Generating Antibiotic Incentives Now), принятой в США в 2012 г. для поддержки разработки новых антимикробных препаратов. Данная инициатива позволяет FDA регистрировать препараты на основании результатов клинических исследований с дизайном «noninferiority» (неменьшей эффективности). Именно 2 исследования с таким дизайном и послужили основанием для одобрительного решения агентства в отношении далбаванцина. Дизайн «noninferiority» позволяет регуляторному органу быстрее внедрять новые препараты в практику, однако подвергается критике в связи с тем, что ограничивает сведения о его безопасности [39].
Далбаванцин
Далбаванцин является полусинтетическим липогликопептидом. По химической структуре он близок к тейкопланину. Название «липогликопептид» далбаванцин, так же как находящиеся в разработке другие препараты этой группы (оритаванцин, телаванцин), получил благодаря наличию в структуре боковой липофильной цепи. Механизм его действия связан с угнетением трансгликозилирования (D-аланил-D-аланин) в клеточной стенке бактерий, что приводит к нарушению ее синтеза [1]. Кроме того, он способен прикрепляться боковой липофильной цепью к мембране бактерий, что приводит к повышению его сродства к мишени и увеличению антимикробной активности.
Препарат обладает высокой активностью в отношении грамположительных кокков, включая стафило-, стрептои энтерококки (минимальная ингибирующая концентрация – МИК90 ≤ 0,25 мкг/мл), вне зависимости от наличия у них резистентности к пенициллину или метициллину [42]. Однако он не активен в отношении резистентных энтерококков, содержащих гены VanA или VanB [42].
По бактерицидной активности in vitro в отношении резистентных грамположительных микроорганизмов он превосходит ванкомицин и тейкопланин [43]. МПК90 далбаванцина в отношении как MSSA, так и MRSA составляют ≤ 0,25 мкг/мл [44].
Кроме того, далбаванцин обладает антианаэробной активностью. МИК в отношении Clostridium difficile, Clostridium perfringens, Peptostreptococcus и Corynebacterium spp. составляет 0,5 мкг/мл или меньше [42]. Резистентность к далбаванцину в настоящее время не описана.
Далбаванцин предназначен для внутривенного введения. Его период полувыведения составляет 9–2 дня, что позволяет вводить препарат 1 раз в неделю и таким образом избегать применения внутривенных катетеров [45–47]. Курс лечения при инфекциях кожи и мягких тканей состоит из двух инъекций – 1000 мг в 1-й день и 500 мг на 8-й.
In vivo далбаванцин проявляет концентрационно-зависимую активность. Фармакодинамическим параметром, наиболее полно определяющим его активность, является соотношение площади под кривой «концентрация– время» (AUC – Area under the curve) к МПК [43, 49].
В клинических исследованиях препарата, представленных в FDA (DISCOVER 1 и DISCOVER 2), приняли участие 1289 пациентов [48]. В обоих исследованиях проведено сравнение далбаванцина (2 инъекции) с внутривенным ванкомицином (не менее 3 дней с последующей возможностью переключения на пероральный линезолид для завершения 10–14-дневного курса). Конечной первичной точкой было улучшение более чем на 20% в очаге инфекции, преимущественно целлюлита, что в соответствии с регуляторными нормами коррелирует с исходами лечения [49]. Оба исследования продемонстрировали равную эффективность препаратов сравнения у пациентов с инфекциями кожи и мягких тканей (83 против 81,8% и 76 против 78,3% соответственно) [48]. Вторичной конечной точкой было клиническое состояние пациентов в конце исследования и оценка исхода лечения исследователями. По оценке последних, клинический успех был достигнут 90,6% участников группы далбаванцина и 93,8% – группы ванкомицина/линезолида.
Еще в одном большом исследовании с участием 854 пациентов с осложненными инфекциями кожи и мягких тканей, вызванных грамположительными микроорганизмами, в т.ч. MRSA, показана «неменьшая эффективность» далбаванцина (2 инъекции) по сравнению с 14-дневными курсами лечения внутривенным или пероральным линезолидом [49].
В исследованиях II фазы далбаванцин по эффективности не уступал цефазолину для пациентов с неосложненными инфекциями кожи и мягких тканей и ванкомицину для пациентов с бактериемией, вызванной S. aureus. Однако результаты этих исследований FDA не представлены [47].
В целом препарат изучался в достаточно большом количестве исследований, однако результаты большинства из них не были опубликованы и не доступны широкой публике [42].
Наиболее частыми побочными эффектами далбаванцина в исследованиях II и III фаз были тошнота, диарея, запор, кандидоз ротовой полости, зуд, головная боль и пирексия [42]. В целом его профиль безопасности не отличался от такового ванкомицина и линезолида как по типу, так и по тяжести нежелательных реакций (НР). В исследованиях DISCOVER-1 и DISCOVER-2 частота НР в группе далбаванцина была даже ниже, чем в группе ванкомицина/линезолида [48]. Серьезные НР наблюдались у 2,6% пациентов в группе далбаванцина по сравнению с 4,0% в группе сравнения.
В некоторых исследованиях имели место летальные исходы, однако ни в одном из случаев смерть не связали с применением препарата [42, 48]. Тем не менее в связи с длительной циркуляцией препарата в организме после его однократного введения безопасность вызывает опасения, т.к. в случае возникновения серьезной НР, в т.ч. аллергической, ее трудно будет купировать [42, 50]. У пациентов с нарушением функции почек возможно повышение уровня ферментов печени, поэтому в инструкцию по применению далбаванцина будет внесено предостережение о необходимости коррекции дозы этим пациентам. Кроме того, есть сообщения об изменении показателей печеночных тестов у пациентов с исходными заболеваниями печени [51]. По-видимому, таким пациентам перед введением второй дозы препарата необходимо тестировать функцию печени [39]. Так же как ванкомицин, далбаванцин обладает низким потенциалом лекарственных взаимодействий.
По мнению экспертов, преимуществами далбаванцина перед ванкомицином являются его активность в отношении некоторых ванкомицинрезистенных штаммов грамположительных микроорганизмов, более низкий потенциал индуцирования бактериальной резистентности, концентрационнозависимая активность, отсутствие необходимости установления внутривенных катетеров и мониторинга концентраций препарата в крови [50].
Все это в сочетании с длительным периодом полувыведения позволяет применять далбаванцин при тяжелых инфекциях кожи и мягких тканей, включая инфекции, вызванные MRSA, в амбулаторных условиях, что может значительно снизить стоимость лечения. В то же время, несмотря на хорошую переносимость, продемонстрированную в клинических исследованиях, эксперты высказывают опасения в отношении безопасности препарата и предостерегают от его применения по незарегистрированным показаниям, например бактериемии или пневмонии [50, 52]. По-видимому, соотношение польза/риск далбаванцина будет определяться при его применении в широкой медицинской практике. Кроме того, сходный с гликопептидами механизм действия препарата не позволит решить проблему лечения инфекций, вызванных ванкомицинрезистентными микроорганизмами. Таким образом, разработка препаратов с принципиально новым механизмом действия для терапии инфекций, вызванных MRSA, особенно VRSA, остается актуальной задачей.
В настоящее время с этой целью изучаются другие липогликопептиды (оритаванцин и телаванцин), представитель нового класса антибиотиков аминометилциклинов омадоциклин, а также «старые» антибактериальные препараты фосфомицин и фузидиевая кислота [44]. Недавно FDA одобрила новый препарат группы оксазолидинонов, тедизолида фосфат, для лечения инфекций кожи и мягких тканей, вызванных грамположительными микроорганизмами, включая MRSA [53].



