Актуальность
Медикаментозная терапия (МТ) занимает особое место среди существующих методов лечения акромегалии (АМ) и активно применяется в клинической практике в качестве вторичной, а в ряде случаев и первичной фармакотерапии. Благодаря постоянному совершенствованию используемых лекарственных форм даже при жизни одного поколения произошли кардинальные изменения в результативности оказания лекарственного пособия: от интуитивной паллиативной помощи, облегчающей страдания больного, к значительно более эффективному синдромальному лечению, далее - к эре персонализированной таргетной терапии, гарантирующей пациенту нормализацию гормонального статуса, повышение качества и продолжительности жизни [1, 2].
На современном этапе эффективность лечения синдрома АМ нельзя рассматривать в отрыве от существующего многообразия патоморфологических форм соматотрофных опухолей, различающихся по рецепторному фенотипу, клиническому сценарию, пролиферативной активности и чувствительности к предъявляемому лечению. Патоморфологи выделяют как минимум 4 морфологических подтипа соматотрофных аденом: плотно гранулированные, редко гранулированные, моноцеллюлярные маммосоматотрофные и бицеллюлярные соматолактотрофные аденомы. В отличие от нормальных соматотрофов эти аденомы преимущественно экспрессируют 2-й и 5-й подтипы соматостатиновых рецепторов (ССР), соотношение между которыми определяет чувствительность или резистентность к лечению аналогами соматостатина (АС) [3, 4].
Современное медицинское пособие при АМ включает транссфеноидальную аденомэктомию, вторичную (первичную) фармакотерапию, лучевое лечение и цитостатическую терапию. Хирургическое лечение является средством выбора, но не всегда успешным, поскольку у большинства пациентов при постановке диагноза имеется инвазивная макроаденома, возможность радикального удаления которой составляет около 40-50%. К предикторам низкого оперативного контроля относятся большие размеры опухоли, инвазия в кавернозный синус, существенно повышенные уровни гормона роста (ГР) и инсулиноподобного ростового фактора-1 (ИФР-1), молодой возраст пациентов, наличие генетических нарушений [1, 5]. Поэтому у половины прооперированных больных сохраняется активность заболевания, требующая подключения вторичной терапии, характер которой в немалой степени зависит от результатов иммуногистохимического анализа (ИГА) удаленного материала, определяющего рецепторный фенотип и пролиферативную активность опухолевых клеток. Как правило, для супрессии активности резидуальной опухоли и достижения контроля АМ используется разнонаправленная МТ, которая включает аналоги соматостатина 1-го и 2-го поколений (АС1 и АС2), неселективные и селективные агонисты дофамина, а также пэгвисомант (ПЭГ), который представляет собой генноинженерный антагонист рецепторов ГР [6, 7].
Согласно международным рекомендациям, препаратами 1-й линии являются АС1 (пролонгированные формы октреотида и ланреотида), преимущественно воздействующие на 2-й подтип ССР и обладающие антисекреторным и антипролиферативным действиями. Наилучшие результаты наблюдаются при лечении больных плотно гранулированными аденомами (ПГА), клетки которых экспрессируют 2-й подтип ССР. Описана прямая корреляция между биохимической чувствительностью к АС1 и уменьшением объема опухолевой ткани. Сравнительные результаты использования пролонгированных форм октреотида и ланреотида больными АМ показали сходные характеристики. В неселективной группе биохимический контроль и уменьшение объема опухолевой ткани, по данным разных авторов, достигаются примерно в 40-50 % случаев. Напротив, редко гранулированные аденомы (РГА) отличаются резистентностью к АС1, повышенной пролиферативной активностью и склонностью к рецидивированию. Особо следует отметить, что РГА включены в список опухолей высокого риска агрессивного течения и малигнизации [8-11].
Второе поколение АС представлено полирецепторным таргетным препаратом пасиреотид ЛАР, обладающим способностью преимущественно воздействовать на 5-й подтип ССР и отличающимся более выраженным антитуморозным действием. Среди побочных эффектов при приеме продленной формы пасиреотида следует отметить высокий риск нарушения углеводного обмена, что ограничивает его широкое применение [12, 13]. В России данная лекарственная форма не зарегистрирована.
Агонисты дофаминергических рецепторов также воздействуют на опухолевые клетки, вызывая парадоксальное снижение секреции ГР. Терапевтические нишы для использования селективного агониста дофамина каберголина: невысокая активность заболевания, наличие смешанных (сомато-лактотрофных) опухолей и частичная резистентность к АС. По данным J. Bollerslev et al., при комбинации каберголина с АС1 безопасный уровень ГР и ИРФ-1 достигается в 75 и 55% случаев [10, 14].
Сравнительно новым инновационным лекарственным средством является антагонист рецепторов ГР ПЭГ, представляющий собой пегилированный генетически модифицированный аналог человеческого ГР с 9 аминокислотными заменами. В силу проведенных модификаций препарат обладает высокой селективностью в отношении рецепторов ГР и путем конкурентного ингибирования блокирует трансдукцию сигнала, обеспечивая стойкое снижение концентрации ИФР-1 и его фракций. Благодаря процессу пегилирования период полувыведения лечебной молекулы из организма увеличен до 74-172 часов, что обеспечивает стабильность терапевтического эффекта ПЭГ. Характерной особенностью препарата является его генерализованное влияние на соматические тканевые рецепторы и независимость действия от конкретного морфотипа соматотрофной опухоли. Ведущим биохимическим маркером для мониторирования эффективности ПЭГ является уровень ИФР-1. В европейских странах препарат используется с 2002 г. у пациентов, не достигших контроля при хирургическом, лучевом или медикаментозном лечении. В ряде случаев (в отсутствие перспективы хирургического или лучевого лечения) его применяют в качестве первичной МТ [15, 16].
Первые клинические исследования показали, что применение ПЭГ способствует нормализации уровня ИФР-1 95% больных, резистентных к АС [17, 18]. В реальной клинической практике контроль АМ был несколько ниже по сравнению с клиническими исследованиями и не превышал 60—70%, что объясняется различиями в целевом уровне ИФР-1 индекса (ИИ), недостаточной терапевтической дозой препарата и низкой приверженностью лечению [10]. Согласно итоговым данным 10-летнего международного обсервационного исследования ACROSTUDY с участием 2221 пациента, контроль АМ на фоне моно- или комбинированной терапии ПЭГ по разным национальным когортам наблюдался в 61-72% случаев, составив в среднем 62%. Биохимическая ремиссия сопровождается уменьшением коморбидности, улучшением показателей углеводного обмена, снижением кардиоваскулярного риска, увеличением толерантности к физической нагрузке [16, 19].
Согласно клиническим наблюдениям, чувствительность к ПЭГ как второй линии терапии зависит от возраста диагноза, индекса массы тела (ИМТ), базального уровня ИФР-1. Как правило, стартовая доза и скорость титрации более высокие у молодых пациентов, у лиц с избыточной массой тела, а также у больных с нарушением углеводного обмена. Предварительные исследования показали, что нормализация ИФР-1 у мужчин требует меньших доз по сравнению с женщинами и у облученных пациентов по сравнению с необлученными больными. Уровень ГР при лечении ПЭГ не является критерием адекватности его применения, однако резкое повышение его уровня может указывать на продолженный рост опухоли [8, 18].
Применение ПЭГ рекомендуется больным АМ с агрессивным течением заболевания после нерадикальной аденомэктомии, неэффективной лучевой терапии, при наличии резистентности к АС 1-й и 2-й генераций. Своевременное включение препарата в лечебную схему позволяет избежать повторного оперативного вмешательства, радиохирургического лечения или назначения избыточно высоких доз АС [1, 16].
Практикуемые лечебные схемы включают комбинированное использование ПЭГ с АС1 или АС2, агонистов дофамина, а также монотерапию. В 2018 г. ПЭГ зарегистрирован в России под торговым названием «Сомаверт» для лечения пациентов с АМ, у которых отсутствовал адекватный ответ на хирургическое лечение (и/или лучевую терапию) и у которых высокодозное лечение АС1 не способствовало нормализации концентрацию ИРФ-1, или при непереносимости данной группы лекарственных средств. В настоящее время ПЭГ активно апробируется в России в качестве моно- или комбинированной фармакотерапии [20].
Цель исследования: оценка эффективности использования ПЭГ в качестве препарата 2-й линии у пациентов с нерадикальной аденомэктомией и резистентностью к АС1.
Методы
В исследование были включены 43 пациента (19 мужчин и 24 женщины). Средний возраст составил 49±14 лет (M±s) в диапазоне от 23 до 74 лет, средний возраст диагноза - 41±12 лет, длительность лечения АМ - 8,0±7,3 года, объем аденомы гипофиза - 2,7±5,4 см3. Исходный уровень ИФР-1 при постановке диагноза составил 833±297 нг/мл, исходная величина ИИ (частное от деления концентрации ИФР-1 на показатель верхней возрастной нормы - ИФР-1/ВВН) - 3,2±1,3. Ранее хирургическое пособие проводилось 38 (88%) пациентам (транссфеноидальная аденомэктомия), четверым из них - повторно. Лучевая терапия (включая стереотаксическую радиохирургию) назначалась 9 (21%) больным. Все пациенты на момент включения в клиническую группу получали терапию АС1 (продленные формы октреотида 30 мг внутримышечно 1 раз в 28 дней [6 человек] или ланреотид Аутожель 120 мг подкожно 1 раз в 28 дней [37 человек]) в качестве монотерапии, а также в комбинации с каберголином (12 человек с дозой от 1,5 до 3 мг в неделю). Несмотря на большую длительность фармакотерапии (63±49 месяцев с максимальным интервалом до 178 месяцев), никем из пациентов не была достигнута медикаментозная ремиссия АМ. Среднее содержание ИФР-1 составило 433±171 нг/мл, средняя величина ИИ - 1,9±0,7. У 26 (60%) из 43 пациентов выявлено нарушение углеводного обмена, у 24 (56%) пациентов - избыточная масса тела (ИМТ - 34,0±3,9 кг/м2). Пятнадцати больным проведен ИГА фрагментов удаленной опухоли с целью установления морфологического диагноза, рецепторного фенотипа и пролиферативной активности опухолевых клеток (исследование проводилось в Референс-Центре патоморфологических, иммуногисто-химических и радиологических методов исследований ФГБУ «НМИЦ эндокринологии» МЗ РФ).
Всем включенным в исследование пациентам дополнительно к лечению АС1 назначен ПЭГ (Сомаверт), прием каберголина был отменен. Стартовая доза ПЭГ составила 10-15 мг (нагрузочная доза - 40-60 мг). На фоне лечения каждые 1-3 месяца в зависимости от необходимости титрации дозы проводился динамический контроль уровня ИФР-1, ИИ, печеночных трансаминаз, показателей углеводного обмена. Контроль размеров соматотрофной аденомы проводился каждые 6 месяцев с помощью магнитно-резонансной томографии (МРТ) головного мозга с контрастным усилением. Объем исходной и резидуальной опухоли вычислялся по формуле De Chiro & Nelson. Биохимическая ремиссия АМ регистрировалась при значении ИИ, равном <1. Длительность лечения в настоящий момент составляет от 3 до 21 месяца (у 30 пациентов >6 месяцев).
Статистический анализ производился с помощью пакета программ «Statistica-12». Меры центральной тенденции и дисперсии количественных признаков, имевших приближенно нормальное распределение, представлены в виде среднего арифметического (М) и среднего квадратического отклонения (s). Анализ зависимостей проводился с использованием коэффициента ранговой корреляции Спирмена. Критический уровень значимости при проверке статистических гипотез принимался равным 0,05.
Результаты
Данные предварительного обследования представлены в таблице.
Как следует из таблицы, представленная группа больных исходно отличалась ранним возрастом диагноза, большими размерами опухоли с высокой секреторной активностью и низкой чувствительностью к АС1, что характерно для РГА, отличающихся агрессивным и рецидивирующим течением. Подтверждением вышесказанного являются данные ИГА, указывающие на то, что у 14 (93 %) из 15 пациентов с окончательным морфологическим диагнозом присутствует РГА с высоким значением пролиферативного индекса Ki-67 (13,1±24,7%) и низким соотношением баллов экспрессии 2-го к 5-му подтипам ССР (по шкале IRS), равным 0,8±0,6. Для плотно гранулированных аденом (ПГА) данное соотношение составляет >2,5. Также важно отметить, что величина снижения уровня ИФР-1 через 3 месяца от начала терапии (в %), которая является отрезной точкой для определения эффективности АС1 в долгосрочной перспективе, в данной группе пациентов составила всего 28±21%, что подтверждает наличие резистентности к данному варианту фармакотерапии.
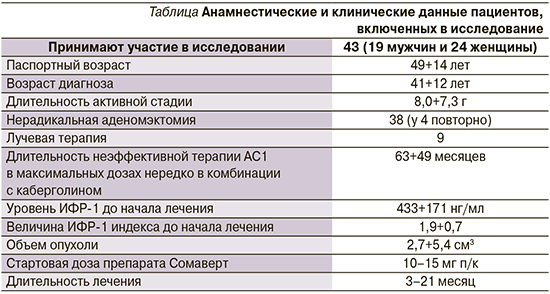
На фоне проводимого комбинированного лечения (АС1+ПЭГ) средний уровень ИФР-1 снизился с 433±171 до 237±91 нг/мл (р=0,0064), величина ИИ - с 1,9±0,7 до 1,1±0,4 (р=0,0089; рис. 1, 2).
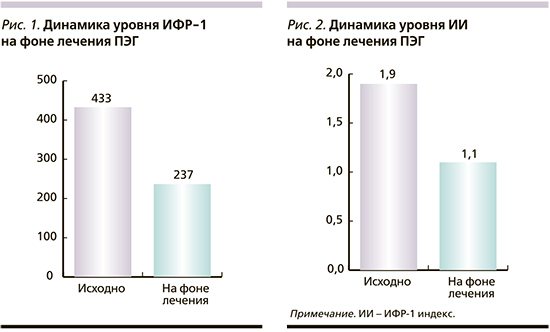
Исходя из промежуточных итогов, во всей группе биохимической ремиссии достигли 56% пациентов, а из 30 больных, получавших лечение >6 месяцев, 17 (57%) в настоящий момент находятся в стадии контроля АМ. В группе пациентов, достигших медикаментозной ремиссии, у семи была снижена терапевтическая доза АС путем увеличения межинъекционного интервала ланреотида Аутожель до 6-8 недель или полной отмены терапии АС1 (рис. 3).
В связи с отсутствием медикаментозной ремиссии 26 пациентам потребовалась титрация дозы с 10 до 15-20 мг/ сут. В настоящее время ПЭГ в дозе 10 мг, 15, 20 мг получают 16, 8 и 16 пациентов соответственно. При этом полная ремиссия наблюдается в 75%, 38 и 50% случаев соответственно. Одна пациентка в настоящий момент получает 30 мг (величина ИИ через 1 месяц с начала использования максимальной дозы препарата составила 1,45 против 3,03 исходно).
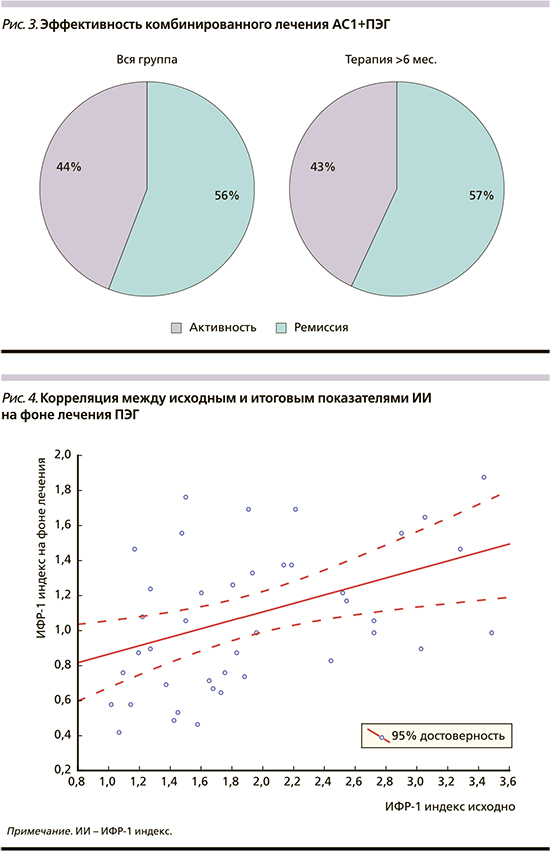
Если говорить о возможных причинах неполной ремиссии АМ у оставшихся 16 (44%) пациентов, то они обусловлены известными и хорошо описанными обстоятельствами, включая исходный высокий уровень ИИ (>2,7) у 4 (25%), наличие нарушен но го углеводного обмена у 13 (81%) и избыточной массы тела у 11 (69%) пациентов, а также превалирование лиц женского пола: 10 (62%). Отмечается прямая корреляция между итоговым уровнем ИИ, с одной стороны, и исходными показателями ИИ (r=0,45; p=0,003), ИМТ (r=0,44; p=0,011), уровнем гликемии (r=0,38; p=0,034) и исходной величиной гликозилированного гемоглобина - HbA1c (r=0,45; p=0,014), с другой (рис. 4-6). Полученные результаты подтверждают данные других исследователей относительно негативного влияния этих факторов на чувствительность к лечению, что требует назначения более высоких насыщающих и стартовых доз ПЭГ [18, 19].
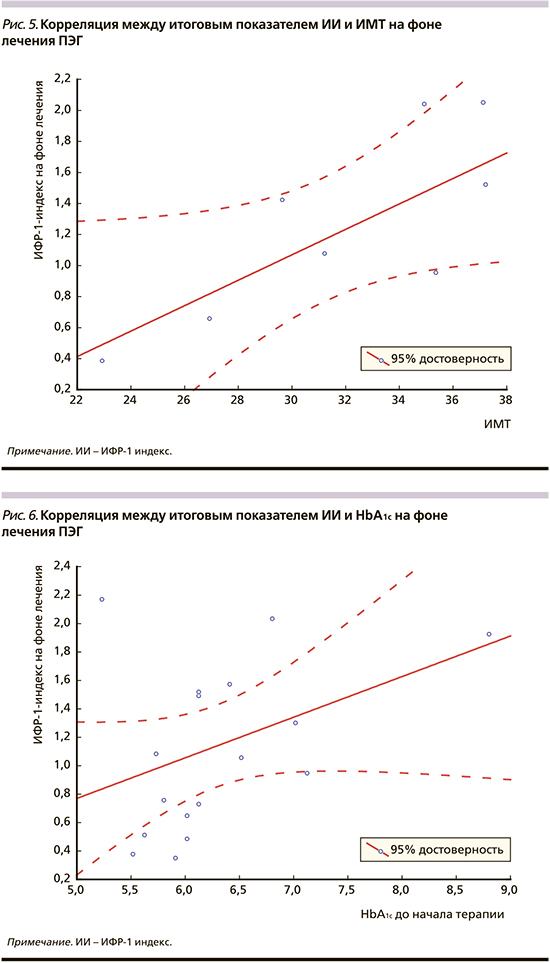
Мета-анализ 13 проспективных исследований, в которых приняли участие 435 пациентов, показал, что пациентам с сахарным диабетом (СД) и ожирением требовалась более высокая средняя доза ПЭГ (18,2 против 15,3 мг/сут). Эти результаты подтверждают более ранний отчет ACROSTUDY, в котором обнаружено: 56 пациентов, которым требовалось >30 мг ПЭГ в сутки для достижения нормализации ИФР-1, были моложе, имели более высокий ИМТ, а также были более склонны к СД и артериальной гипертензии. Эти результаты также свидетельствуют о том, что пациентам с СД и/или избыточной массой тела требуются более высокие стартовые дозы ПЭГ и более активное титрование препарата для нормализации уровня ИФР-1 [19, 21].
В целом, по нашим данным, переносимость препарата была хорошей. За весь период наблюдения у больных отмечалось небольшое увеличение уровней печеночных трансаминаз, не превышавшее референсных значений: аланинаминотрансфераза - 25,7±17,1 против 21,9±14,1, аспартата-минотрансфераза - 25,1±16,3 против 21,8±7,1, Y—глутамилтранспептидаза - 36,0±40,3 против 27,7±21,0 ЕД/л. Лишь в одном случае у пациентки с сопутствовавшим стеатогепатитом выявлено значительное превышение уровня Y-глутамилтранспептидазы 252 против 109 ЕД/л, потребовавшее отмены ПЭГ. По данным M. Fleseriu et al., повышение уровня печеночных трансаминаз на фоне длительного лечения ПЭГ зарегистрировано у 3,2% пациентов [16]. В нашем случае эта величина составила 2,3%.
Что касается динамики показателей углеводного обмена на фоне комбинированного лечения, в целом отмечено незначительное снижение гликемии натощак: 6,3±1,7 против 6,4±1,7 ммоль/л и HbA1c 6,2±1,2 против 6,3±1,1%. Стоит отметить, что отсутствие ухудшения углеводного обмена является отличительной особенностью ПЭГ по сравнению с АС 1-го и особенно 2-го поколения.
Тщательный контроль размеров аденомы гипофиза, по данным МРТ с контрастным усилением, не выявил отрицательной динамики. Объем аденомы гипофиза на фоне комбинированного лечения составил 3,3±5,6 против 3,3±5,0 см3 исходно. Согласно протоколу ACROSTUDY, значимым изменением опухолевого размера является увеличение наибольшего диаметра > 3 мм или опухолевого объема >20%. По данным разных авторов, величина продолженного роста при монотерапии ПЭГ составляет от 3,7 до 7,1% [16-18]. В нашем случае, несмотря на повышенные значения Ki-67, существенных изменений объема опухоли не зарегистрировано. По данным литературы, продолженный опухолевый рост наблюдается в течение 1-го года лечения ПЭГ и может отражать агрессивный характер опухолевого развития или быть следствием прекращения лечения АС (ребаунд-эффект) [16]. В работе A. Bianchi et al., отмечено, что только у 1 из 35 наблюдаемых пациентов выявлен небольшой рост (>25%) резидуальной аденомы, который в дальнейшем не прогрессировал после 6 месяцев лечения. У данного пациента отмечалось агрессивное течение заболевания с плохим контролем, несмотря на активное лечение (40 мг ПЭГ и 120 мг ланреотида Аутожель каждые 4 недели) [22]. Согласно результатам ACROSTUDY, за 10 лет наблюдения продолженный рост имел место лишь в 3,75% случаев (83/2221). Тем не менее, принимая во внимание, что большая часть наблюдаемых нами пациентов, по данным ИГА, имеют РГА, отличающиеся повышенной митотической активностью и высоким риском малигнизации, представляется обоснованным регулярное проведение МРТ резидуальной опухоли для своевременного выявления отрицательной динамики.
Таким образом, за 1,5-годичный период наблюдения у пациентов не было зарегистрировано значительных побочных эффектов, приведших к отмене ПЭГ (кроме 1 случая). Представленные данные согласуются с результатами японского постмаркетингового наблюдения за эффективностью лечения ПЭГ в обычной клинической практике, подтвердившего отсутствие у больных серьезных проблем с безопасностью препарата. Нормализация уровня ИФР-1 за 5 лет лечения ПЭГ достигнута у 53,5% больных (130/243) [23]. Однако стоит отметить, что необходимость ежедневного введения препарата и, соответственно, наличие некоторого обременения для пациентов, возможно, требуют рассмотрения иных терапевтических схем с менее частым введением препарата для повышения приверженности лечению.
Обсуждение
Основные задачи лечения АМ: достижение контроля гиперсекреции ГР, предотвращение последствий масс-эффекта и повышение качества жизни с минимальными побочными эффектами. Согласно консенсусному соглашению, применение ПЭГ в качестве монотерапии (или комбинации с АС1) рекомендуется пациентам, у которых отсутствует чувствительность к АС1, т.е. снижение уровня ИФР-1 составляет менее 20%. В работе E.C. Coopmans et al. рекомендуется комбинация ПЭГ и АС1 в качестве второй линии лечения при всех случаях отсутствия чувствительности, определяемой как сохранение величины ИИ >1,3 [24].
Клинические результаты проекта ACROSTUDY показывают, что поддержание контроля по мере увеличения длительности лечения требовало постепенного повышения дозы препарата (с 14,0 мг за 1-й год до 18 мг/сут на 10-й год лечения), причем более значительного у лиц с сохраняющейся активностью заболевания. Что же касается терапевтических схем, то примерно 53,3% получали ПЭГ в качестве монотерапии, тогда как в остальных случаях применялось комбинированное лечение (ПЭГ+АС и/или агонист дофамина). Последняя тактика активно использовалась пациентами с агрессивным течением АМ, большими размерами опухоли и частичной резистентностью к АС1 [12]. По мнению M. Gadelha et al., комбинация ПЭГ с АС1 позволяет пациентам с частичной резистентностью достигать контроля заболевания в 80% случаев [6, 25].
В ходе проспективного рандомизированного исследования V. Bonert et al., апробированы различные режимы 24—32-недельной комбинированной терапии у 52 больных АМ, резистентных к максимальным дозам АС1. Авторы показали, что предложенная ими комбинация низких доз АС1 (октреотида LAR 10 мг/28 дней) или ланреотида (60 мг/28 дней) в сочетании с еженедельным приемом ПЭГ (40—160 мг в неделю) способствовала достижению биохимического контроля у 96% больных при значительно меньших финансовых затратах по сравнению с другими режимами приема: (октреотид ЛАР 30 мг или ланреотид 120 мг/28 дней+ПЭГ 40-160 мг/нед.) или (октреотид ЛАР 10 мг или ланреотид 60 мг/28 дней+ПЭГ 15-60 мг/сут). Стоимость лечения в среднем составила 9837±1375 против 14261±1645 и 22543±11158 долл. США в месяц соответственно. Таким образом, авторы делают вывод, согласно которому режим низких доз АС1 в сочетании с еженедельным приемом ПЭГ представляет собой новый вариант дозирования для достижения экономически эффективного, оптимального биохимического контроля у пациентов с неконтролируемой АМ, требующих комбинированной терапии [26].
По мнению многих исследователей, комбинированное лечение, воздействующее на различные патогенетические механизмы развития заболевания, обеспечивает более надежный контроль соматотропной функции и уменьшение суммарной дозы препаратов. Поскольку монотерапия ПЭГ не уменьшает объема опухоли, то его комбинация с АС1 блокирует продолженный рост аденомы гипофиза у большинства пациентов. В данном случае играет роль антипролиферативный эффект АС1, который реализуется независимым путем в отличие от антисекреторного действия [1, 27, 28].
Поскольку эффективность ПЭГ как ингибитора рецепторов ГР зависит от его концентрации в крови, действие АС1, направленное на подавление опухолевой секреции ГР, снижает его содержание и уменьшает потребность в препарате. Из сказанного следует, что терапевтическая доза ПЭГ зависит от дозы АС1. Кроме того, следует учитывать и внегипофизарное действие АС1, проявляющееся: а) снижением численности и экспрессии рецепторов ГР в печени в результате уменьшения концентрации инсулина в портальной системе; б) ингибирующим действием на секрецию ИФР-1 гепатоцитами; в) повышением концентрации ПЭГ в крови. Таким образом, прямое и опосредованное действие АС объясняет снижение терапевтической дозы ПЭГ при комбинированном лечении [29].
Ниже представлено два клинических случая использования ПЭГ.
Клинический случай 1
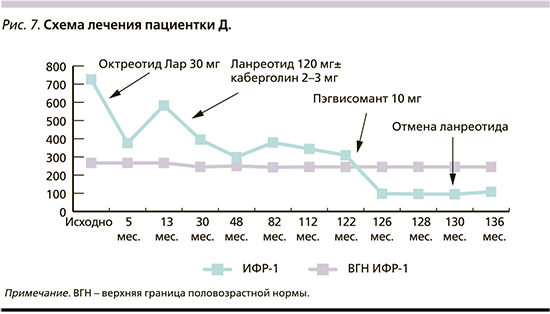
Пациентке Д. 52 лет со стажем АМ 19 лет (исходный уровень ИФР-1 - 511 нг/мл, величина ИИ - 1,21) проведена нерадикальная транссфеноидальная аденомэктомия, после чего назначена фармакотерапия АС1 (октреотид Лар 30 мг/28 дней, ланреотид 120 мг/28 дней) в сочетании с каберголином (до 3 мг/нед.). На этом фоне уровень ИФР-1 сохранялся выше референсных значений (299-583 нг/мл). В августе 2020 г. к лечению был добавлен ПЭГ в дозе 10 мг ежедневно, каберголин был отменен. Через 2 месяца была зарегистрирована медикаментозная ремиссия АМ (ИФР-1 - 98 нг/мл, ИИ - 0,4). В дальнейшем постепенно увеличивался межинъекционный интервал введения ланреотида до 8 недель (ИФР-1 - 95 нг/мл). После получения результатов МРТ гипофиза с контрастным усилением (отсутствие визуализации аденомы) принято решение об отмене ланреотида (рис. 7). На фоне монотерапии ПЭГ величина ИИ составила 0,44.
Клинический случай 2
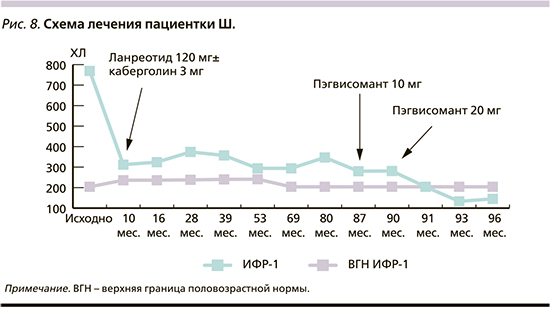
Пациентке Ш. 64 лет со стажем АМ 9 лет (исходный уровень ИФР-1 - 1250 нг/мл, величина ИИ - 3,25; объем аденомы гипофиза - 5,07 см3), в связи с нерадикально проведенной трансназальной аденомэктомией назначена вторичная фармакотерапия ланреотидом 120 мг/28 дней с последующим добавлением каберголина 3 мг/нед., которая не привела к биохимической ремиссии (ИФР-1 - 272-316 нг/мл). В марте 2021 г. к лечению был добавлен ПЭГ в дозе 10 мг ежедневно, каберголин отменен, однако уровень ИФР-1 оставался повышенным (ИИ - 1,38). В июне 2021 г. доза ПЭГ увеличена до 20 мг, что привело к нормализации ИФР-1 уже через месяц (ИИ - 0,91) (рис. 8). Длительность эффективного лечения на данный момент составляет 8 месяцев (величина ИИ - 0,71).
Заключение
Таким образом, на основании полученных результатов можно сделать вывод: подключение ПЭГ к лечению пациентов, плохо контролируемых при высокодозной монотерапии АС1, обеспечивает достаточный биохимический и опухолевый контроль при минимальных побочных эффектах. Дальнейшее накопление клинического материала с апробацией дифференцированных режимов лечения позволит повысить эффективность фармакотерапии и уменьшить суммарную терапевтическую дозу препаратов.


