Введение
Нейтрофилы, известные как полиморфно-ядерные лейкоциты, являются наиболее распространенным типом клеток периферической крови человека. Нейтрофилы созревают в костном мозге в большом количестве (~1011 клеток в сутки) и считаются важными эффекторными клетками врожденного иммунитета. В гомеостатических условиях они попадают в кровообращение, мигрируют в ткани, играют ключевую роль в воспалительных процессах и биологической защите человека. Классическая точка зрения – это мономорфная популяция, однако в настоящее время показано, что нейтрофилы представляют собой транскрипционно активные комплексные клетки, обладающие большой фенотипической гетерогенностью и функциональной универсальностью. Нейтрофилы обнаруживают зону заражения или повреждения, запускают процесс фагоцитоза, дегрануляции, высвобождение ядерного материала в виде внеклеточных ловушек нейтрофилов (от англ. Neutrophil Extracellular Traps); секретируют хемокины и цитокины, мобилизуют моноциты из периферической крови в ткани и способствуют их созреванию в макрофаги, регулируют функцию макрофагов для долгосрочных иммунных реакций, активируют компоненты адаптивного иммунитета, играют роль в формировании клеток иммунологической памяти, активно участвуют в развитии некоторых заболеваний, включая рак [10, 19, 22, 24, 26, 36].
Роль нейтрофилов в онкогенезе
Показано, что нейтрофилы могут участвовать в онкогенезе, включая инициацию опухоли, рост, пролиферацию и метастазирование [9, 35]. Рецепторы нейтрофилов CXCR2 привлекаются лигандами CXCL1, CXCL2, CXCL5 опухолевых клеток и элементами опухолевого микроокружения, играют центральную роль в процессах воспаления внутри опухолевых очагов [9, 23]. Инициации опухоли может способствовать высвобождение нейтрофилами активных форм кислорода (ROS), азота (RNS), протеаз; пролиферации опухоли: перенос нейтрофильной эластазы в опухолевые клетки опосредованной деградацией субстрата рецептора инсулина (IRS1) и активацией PI3K сигнального пути. Следует отметить, что продукция iNOS в нейтрофилах также может стимулироваться путем повышения регуляции рецептора тирозинкиназы MET [11]. Важным механизмом участия нейтрофилов в опухолевом процессе является индукция ангиогенеза с помощью продукции сосудистого эндотелиального фактора роста (VEGFA), прокинетицина-2 (PROK-2) или матриксной металлопротеиназы-9 (ММР-9). Кроме того, нейтрофилы могут стимулировать метастазирование опухоли, подавляя естественную киллерную функцию NK-клеток (NK – от англ. Natural Killer) и облегчая экстравазацию опухолевых клеток. Противоопухолевый ответ CD8+ Т-лимфоцитов может подвергаться угнетению синтазой оксида азота (iNOS) или аргиназой-1 (ARG1), высвобождаемой нейтрофилами при стимуляции их трансформируюшим ростовым фактором-β (TGFβ) [3, 13, 30, 33, 38, 40].
В настоящее время описаны субпопуляции полиморфноядерных нейтрофильных миелоидных супрессорных клеток (от англ. Polymorphonuclear Neutrophils Myeloid-Derived Suppressor Cells), которые в патологических условиях подавляют активацию и пролиферацию Т-лимфоцитов, модулируют противоопухолевый иммунный ответ [37, 39].
Таким образом, роль нейтрофилов в онкогенезе сложная и многокомпонентная: они могут способствовать опухолевому росту, но могут подавлять метастазирование, как это показано для рака легких. Это различие в функциях может быть связано с существованием различных субпопуляций нейтрофилов, в т.ч. при разделении их на субпопуляции N1 и N2 [9, 12, 35].
Интересным оказалось изучение индекса нейтрофилы/лимфоциты (ИНЛ) и роль лимфоцитов в период клеточной кооперации нейтрофилы/лимфоциты, обусловленной опухоль-индуцированной Т-клеточной дисфункцией.
Иммунологические основы анергии Т-лимфоцитов
Известно, что активация и дифференцировка Т-лимфоцитов строго контролируются в зависимости от контекста, в котором «наивные» Т-клетки распознают антиген с образованием эффекторных Т-клеток и Т-клеток-памяти, реализацией генетически запрограммированного иммунного ответа. В то же время активация Т-лимфоцитов может вызывать их дисфункцию, включая истощение, толерантность, анергию или старение. Принято считать, что при прогрессировании опухолевого процесса Т-клетки находятся в функционально «истощенном» состоянии вследствие высокой опухоль-антигенной нагрузки и воздействия иммуносупрессивных факторов микроокружения опухоли, гипотетически характерном для хронической инфекции. Эта гипотеза в значительной степени основана на наблюдении за фенотипическими и функциональными характеристиками «истощенных» Т-клеток при хронических инфекциях и при исследовании опухоль-инфильтрирующих CD8 Т-лимфоцитов (TIL), у которых описано нарушение продукции эффекторных цитокинов, выявление экспрессии ингибирующих рецепторов, включая PD-1, LAG-3, 2B4, TIM-3, CTLA-4, изменение в сигнальных путях, характерных для «истощенных» Т-клеток [14, 28, 29, 34].
Показано, что микроокружение опухоли подавляет митохондриальный биогенез Т-клеток, приводит к внутриклеточной метаболической недостаточности и дисфункции. Геномный транскриптомный анализ Т-клеток Melan-A/Mart-1-specific CD8, выделенных из метастазов больных меланомой, выявил профиль «истощенных» Т-лимфоцитов, соответствующий «истощенным» Т-клеткам мышей в процессе хронической инфекции клона LCMV 13 [5].
Заражение экспериментальных мышей вирусом LCMV-13 определило прототип модели функционального нарушения Т-клеток, называемого истощением Т-лимфоцитов, с прогрессивным снижением продукции интерлейкина-2, фактора некроза опухоли-α, интерферона-γ и неспособностью лизировать инфицированные клетки-мишени.
Анализ «истощенных» Т-лимфоци-тов привел к значительным открытиям, таким как идентификация PD-1, ключевой ингибиторный рецептор, участвующий в основных функциях Т-клеток. Профилирование экспрессии генов мышиных Т-лимфоцитов показало, что «истощение» этих клеток связано с многочисленными молекулярными изменениями, влияющими на гены, регулирующие экспрессию ко-рецепторов, хемотаксис, адгезию, миграцию, метаболизм и энергетические процессы. В совокупности эти изменения характеризовали прогрессирующую потерю эффекторных функций Т-лимфоцитов, высокую и устойчивую экспрессию ингибирующих рецепторов, метаболическую дисрегуляцию, нарушение формирования иммунологической памяти, гомеостатического самообновления, обновления транскрипционных и эпигенетических программ, приобретение профиля функционального истощения Т-лимфоцитов [20].
Недавно установлено, что функцио-нально истощенные Т-клетки – это гетерогенная лимфоидная популяция, включающая субпопуляции с уникальными характеристиками и разнообразными ответами на блокаду контрольных точек иммуноонкологическими препаратами. Несомненно это обусловлено биологическими особенностями организма, формированием иммунного ответа на опухоль, заложенными в фармакокинетике иммуноонкологических препаратов. В то же время при изучении фармакодинамики, предиктивных или прогностических биомаркеров были выявлены уникальные проблемы нашего незнания многих аспектов формирования противоопухолевого иммунного ответа [31].
В связи с этим важным становится характеристика иммуногенных и неиммуногенных опухолей, опухолевого микроокружения в контексте использования иммуноонкологических препаратов и оценки ИНЛ в клинической практике. В иммуногенной опухоли выявляются многие иммунологические маркеры, в т.ч. CD8- и CD4-Т-лимфоциты, PD-L1, гранзим Б и CD45RO (Т-клетки памяти), способствующие эффективному разрушению опухоли под воздействием антител к ингибиторам контрольных точек (анти-PD-1, анти-CTLA-4 и др.). Опухоли, у которых отсутствует экспрессия большинства ключевых иммунологических маркеров, указывают на неиммуногенное микроокружение и требуют комбинированной терапии, в которую входят агент(ы), способствующие созданию иммуногенного микроокружения.
В настоящее время становится понятным, что для достижения устойчивого клинического эффекта в лечении злокачественных новообразований необходимо наличие или образование иммуногенного опухолевого микроокружения в процессе «стандартного» и экспериментального лечения, которое может способствовать длительным клиническим эффектам и излечению от рака.
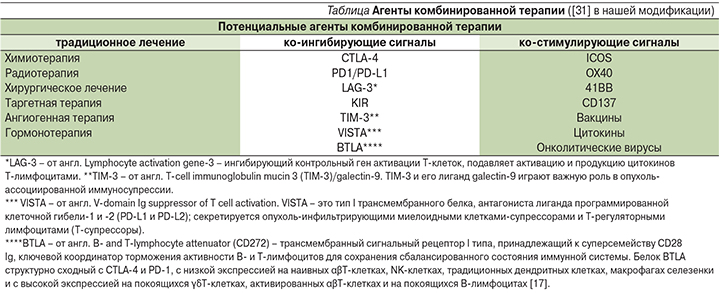
Потенциальные агенты комбинированной терапии в нашей модификации представлены в таблице.
Клиническое применение ИНЛ
Многократно описано увеличение ИНЛ в ассоциации с прогрессированием опухолевого процесса, чувствительностью к химиотерапии, лучевой терапии, токсичностью, с неблагоприятным прогнозом для некоторых нозологических форм злокачественных новообразований [6, 7, 25, 32].
В то же время ИНЛ как предиктивный маркер эффективности иммунотерапии, комбинированной иммунотерапии иммуноонкологическими препаратами, активации ко-стимулирующих сигналов в контексте изучения иммуногенного и неиммуногенного опухолевого микроокружения, гетерогенной популяции нейтрофилов и «истощенных» Т-лимфоцитов в настоящее время представлен фрагментарно в ограниченном числе публикаций.
Вместе с тем D.B. Sacdalan et al. (2018) [27] провели мета-анализ по результатам опубликованных исследований и показали, что ИНЛ до лечения коррелировал с другими биомаркерами, отражал элементы системного воспаления, связанного с прогнозом при сóлидных опухолях и терапии ингибиторами иммунных контрольных точек (ИКТ). Прямая корреляция была обнаружена для ИНЛ и внутриопухолевых нейтрофилов миелоидного супрессорного гистогенеза. Повышение уровня Т-регуляторных лимфоцитов периферической крови коррелировало с увеличением ИНЛ и ассоциировалось с неблагоприятным прогнозом для пациентов с раком поджелудочной железы.
Регрессионный анализ показал, что пожилые пациенты (74,00±6,58 года) лучше отвечают на лечение PD-1-ингибиторами (ОР=0,226; p=0,01) в отличие от молодых больных (59,00±6,32 года; р<0,001). Эта закономерность отмечена и для ИНЛ: у пожилых пациентов он был ниже (2,84±0,90) по сравнению с молодыми больными (4,26±2,25; р<0,01) и был независимым фактором при разных типах опухолей [4].
Изменение ИНЛ в ответ на терапию ИКТ описано для метастатического почечно-клеточного рака. В исследовании A-K.A. Lalani et al. (2018) медиана наблюдения для этой категории больных составила 16,6 месяца (диапазон: 0,7–67,8). Медиана продолжительности терапии – 5,1 месяца (<1–61,4). Группы риска по критериям IMDC (IMDC от англ. International Metastatic Renal Cell Carcinoma Database Consortium) были: 18% – благоприятный риск, 60% – промежуточный, 23% – плохой. Пациенты находились на 1-й, 2-й и более линиях терапии. Медиана ИНЛ составила 3,9 (1,3–42,4) до лечения анти-PD-1/PD-L1 и 4,1 (1,1–96,4) на шестой неделе. У пациентов с более высоким исходным уровнем ИНЛ была обнаружена тенденция к более низкому полному или частичному ответу, более короткому времени до прогрессирования (ВДП) и низкой общей выживаемости. Более высокий ИНЛ на 6-й неделе наблюдения был статистически значимым предиктором для всех 3 групп риска по сравнению с ИНЛ до лечения анти-PD-1/PD-L1. Относительное изменение ИНЛ≥25% от исходного уровня на 6-й неделе терапии ассоциировалось со снижением частичного ответа и было независимым прогностическим фактором для ВДП (р<0,001) и общей выживаемости (р=0,004), в то время как снижение ИНЛ≥25% было ассоциировано с лучшим ответом на терапию ИКТ [16].
Заключение
Таким образом, ИНЛ становится доступным биомаркером в рутинной клинической практике. Он может использоваться в дополнение к другим прогностическим факторам и новым генетическим предикторам. ИНЛ может использоваться для совершенствования терапевтических алгоритмов и принятия решений в отношении целесообразности проведения иммунотерапии ИКТ и ее сочетания с потенциальными агентами комбинированной терапии. Важно следующее: ИНЛ – легковыполнимый и дешевый прогностический фактор, который может улучшать результаты различных методов иммунотерапии онкологических больных в процессе изучения функциональной активности различных субпопуляций нейтрофилов и лимфоцитов как в опухолевом микроокружении, так и в периферической крови.


