Введение
Рак легкого занимает первое место в структуре смертности от злокачественных новообразований. Немелкоклеточный рак легкого (НМРЛ) – крайне гетерогенное заболевание как с морфологической и молекулярно-генетической, так и с клинической точки зрения, что определяет сложность алгоритмизации всех применяемых в лечении подходов [1]. Так, для различных стадий этого заболевания могут применяться все существующие методы противоопухолевого воздействия: хирургический, лучевая и лекарственная терапии [2, 3]. Кроме того, при раке легкого продемонстрирована целесообразность использования практически всех видов лекарственной терапии: цитостатической, таргетной и терапии ингибиторами иммунных контрольных точек – ИИКТ [4–6].
Разработка новых противоопухолевых препаратов, способных потенцировать противоопухолевую активность иммунной системы, резко изменила подходы к лечению онкологических заболеваний в целом и рака легкого в частности. Так, в крупных рандомизированных исследованиях преимущество эффективности монотерапии ИИКТ относительно стандартного лечения цитостатическими препаратами была определена сначала для 2-й линии системной лекарственной терапии НМРЛ. В исследование CheckMate-057 включались пациенты после регистрации прогрессирования опухолевого процесса на фоне или после терапии платиновыми дуплетами. Проводимая терапия разделялась на два рукава: в одном – стандартная терапия доцетакселом 75 мг/м2, во втором – терапия ниволумабом 3 мг/м2 1 раз в 2 недели. Результаты данного исследования продемонстрировали преимущество иммунотерапии (ИТ), что отображено в статистически значимом увеличении медианы общей выживаемости (мОВ) с 9,4 до 12,2 месяца (доверительный интервал [ДИ]: 95% 9,7–15,0; отношение рисков [ОР]=0,73, 96% ДИ от 0,59 до 0,89; р=0,002) [7]. Терапия ИКТ проводилась до прогрессирования заболевания или непереносимой токсичности. Вопрос о целесообразности подобного подхода к длительности лечения был поднят в исследовании CheckMate-153, в котором проведено сравнение сокращенного курса ИТ на протяжении фиксированного периода времени (1 год), относительно стандартного по длительности – до прогрессирования заболевания или развития непереносимой токсичности.
В результате продемонстрировано преимущество непрерывного лечения. При минимальном последующем наблюдении после рандомизации 13,5 месяцев медиана выживаемости без прогрессирования (мВБП) была боль-ше при непрерывном лечении по сравнению с 1-летним лечением фиксированной продолжительности: ВБП: 24,7 против 9,4 месяца (ОР=0,56, 95% ДИ: 0,37–0,84) [8].
Важно отметить, что значимость исследования CheckMate-057 заключалась не в довольно скромном увеличении мВБП, а в том, что для части пациентов, а именно для 13,4% группы терапия ингибиторами PD-1/PD-L1 позволяла достигать пятилетней и более выживаемости против 2,6% в группе химиотерапии (ХТ) [9]. Одним из факторов, позволившим значительно увеличить показатели выживаемости больных НМРЛ, чувствительных к действию ИКТ, стал высокий уровень экспрессии PD-L1.
Экспрессия PD-L1 определяется методом иммуногистохимии. Оценка осуществляется путем подсчета соотношения опухолевых клеток с позитивным окрашиванием мембраны к общему числу опухолевых клеток (TPS – tumor proportion score), результат выражается в процентах от 0 до 100. По уровню PD-L1 опухоли легкого разделяются на три группы: высокий (≥50%), средний (1–49%) и низкий уровень (<1) [10].
Несколько позднее исследование KEYNOTE-024 выявило существенное преимущество моноиммунотерапии (мИТ) относительно ХТ препаратами платины в группе пациентов с высоким уровнем экспрессии PD-L1 в 1-й линии лечения. Применение пембролизумаба достоверно снизило относительный риск прогрессирования на 50% и риск смерти на 37% по сравнению с ХТ. Медиана продолжительности жизни в группе ИКТ достигла 30,0 месяцев по сравнению с 14,2 в группе ХТ при одновременной лучшей переносимости лечения. В связи с этим пембролизумаб рекомендован в качестве предпочтительной 1-й линии системной терапии больных НМРЛ с высокой экспрессией PD-L1, что отображено во всех клинических рекомендациях [11, 12]. Несмотря на очевидное преимущество в эффективности данной терапии, вопрос о продолжительности лечения ИКТ в рамках 1-й линии системной лекарственной терапии по-прежнему остается неизученным и составляет 2 года, что соответствует длительности лечения регистрационного исследования, однако никаких дальнейших исследований по изучению данного вопроса на сегодняшний день не проведено.
Цель исследования. Оценить показатели эффективности мИТ в 1-й линии лечения НМРЛ: частоту объективных ответов (ЧОО), ВБП, общую выживаемость (ОВ) пациентов, терапия которых была прекращена по организационным или другим причинам.
Методы
Был проведен ретроспективный анализ базы данных, включившей пациентов, проходивших лечение на базе ГБУЗ «Санкт-Петербургский клинический научно-практический центр специализированных видов медицинской помощи (онкологический)» в 2019–2020 гг.
Основным критерием отбора пациентов для текущего анализа было применение монотерапии пембролизумабом в 1-й линии системной терапии неоперабельного НМРЛ. Выбор в его пользу основывался на следующих критериях: уровень экспрессии PD-L1>/=50% независимо от общесоматического статуса и сопутствующей патологии у пациентов с неагрессивным течением заболевания или уровень экспрессии PD-L1 0–49% при наличии выраженной сопутствующей патологии и/или общесоматический статус по ECOG (Eastern Cooperative Oncology Group) 2–3, что стало противопоказанием к назначению цитостатической терапии. Другие критерии, учтенные при определении тактики лечения, – это ожидаемая продолжительность жизни не менее 2 месяцев, адекватная гемопоэтическая функция на момент начала лечения, адекватная функция печени, почек. Допускались пациенты с наличием сопутствовавшей патологии (хроническая сердечная недостаточность [ХСН] 2-го функционального класса по NYHA и выше, хроническая обструктивная болезнь легких [ХОБЛ] средней/тяжелой степени тяжести), с наличием метастатического поражения головного мозга без неврологического дефицита. Не включались пациенты с наличием в анамнезе активного аутоиммунного заболевания, для лечения которого применялись системные глю-кокортикостероиды в течение последних 6 месяцев до начала терапии.
Оценка иммуногистохимического (ИГХ) статуса PD-L1 производилась в соответствии с рутинными процедурами центра с использованием антител DAKO-22C3 или VENTANA PD-L1 (SP263). До начала терапии больным проводилось обследование с целью оценки распространения заболевания: компьютерная томография (КТ) грудной клетки и брюшной полости с внутривенным (в/в) контрастированием, магнитно-резонансная томография головного мозга (МРТ) с в/в контрастированием.
Терапия пембролизумабом проводилась в стандартном режиме в дозе 200 мг внутривенно капельно каждый 21 день. Для оценки переносимости и токсичности терапии перед каждым циклом проводились объективный осмотр, сбор жалоб, анализ клинико-лабораторных показателей крови давностью не более 5 дней.
Оценка эффективности лечения производилась в соответствии с рутиной клинической практикой – методом спиральной КТ грудной клетки, брюшной полости с в/в контрастированием и МРТ головного мозга с в/в контрастированием. Эффект проводимой терапии оценивался в соответствии с критериями RECIST (Response evaluation criteria in solid tumours) 1.1 [13]. ВБП на фоне 1-й линии терапии рассчитывалось от первого дня первого цикла терапии до регистрации прогрессирования по данным очередной КТ, появления клинических признаков прогрессирования заболевания или смерти, если она была констатирована без подтверждения прогрессирования болезни объективными методами. ОВ больных рассчитывалась от первого дня первого цикла 1-й линии терапии до даты его смерти или даты последнего контакта с пациентом.
Отмена терапии проводилась в следующих случаях: прогрессирования болезни, непереносимой токсичности, отказа больного от дальнейшего лечения или решения врача о прекращении терапии.
В связи с неконтролируемым распространением новой коронавирусной инфекции 11 марта 2020 г. Всемирной организацией здравоохранения (ВОЗ) была объявлена пандемия, вызванная SARS-CoV-2 [14]. Первый случай коронавирусной инфекции в Санкт-Петербурге был зарегистрирован 5 марта 2020 г., а введение карантинных мероприятий осуществлено начиная с 16 марта 2020 г. Последующие ограничительные меры были оглашены 23 марта 2020 г.: установлен запрет на плановые госпитализации, посещение медицинских организаций амбулаторного типа, диспансеризацию, вакцинацию. На этом фоне продолжение планового лечения, основанного на госпитализациях пациентов каждые 3 недели, было затруднено, в связи с чем части пациентов с показанием к продолжению ИТ в рамках 1-й линии системной лекарственной терапии НМРЛ лечение было приостановлено.
Результаты
В ходе ретроспективного анализа были отобраны 230 больных НМРЛ, получавших 1-ю линию системной лекарственной терапии за 2019–2020 гг. на базе ГБУЗ «СПбКНПЦ СВМП(о)». Из них 104 пациента получали комбинированную ХТ на основе препаратов платины, 39 – химиоиммунотерапию, монотерапию пембролизумабом – 25 пациентов, которые и вошли в данный анализ. CONSORT-диаграмма включения больных в исследование пред-ставлена на рис. 1.
Клинические характеристики больных, получавших мИТ в рамках 1-й линии лечения, представлены в таблице.
Уровень PD-L1 большинства пациентов, а именно 18 (72%), получавших пембролизумаб в 1-й линии, оценивался как высокий (>/=50%), лишь у 3 (12%) был средний уровень (1–49%), негативный (0%) у 1 (4%), не оценен у 3 (12%).
Выбор пациентов со средним и низким PD-L1-уровнем экспрессии в пользу мИТ осуществлялся в случае наличия у них выраженной сопутствовавшей патологии, при наличии ХСН 2-го функционального класс по NYHA и выше, ХОБЛ средней/тяжелой степени тяжести, общесоматическом статусе по ECOG 2–3. Проведение им ХТ сопряжено с крайне высоким риском развития серьезных нежелательных явлений или с декомпенсацией сопутствовавших заболеваний.
Среднее число введений пембролизумаба составило 9,5 (1–28). Эффект лечения был оценен в отношении 18 (72%) пациентов. Частичный регресс зафиксирован у 8 (32%), полный – у 3 (12%), стабилизация – у 7 (28%). Среднее время наблюдения составило 13,1 (0,8–26) месяца. Медиана ВБП на момент проведения анализа не была достигнута, 6-месячное ВБП – 67%.
Прогрессирование на фоне терапии зафиксировано у 12 (48%) на фоне среднего числа проведенных циклов 4,1 (1–16), 2 пациента погибли после 1-го цикла, у 1 из них зарегистрировано гиперпрогрессирование. Таким образом, продолжение лечения до 2 лет требовалось 13 пациентам.
На фоне пандемии SARS-CoV-2 и мероприятий по профилактике его распространения лечение было приостановлено в отношении 9 (69%) из 13 пациентов: у 6 (67%) – из-за ужесточения требований эпидемиологической ситуации, у 3 (33%) – из-за отказа от посещения медучреждений.
На фоне терапии у 4 (44%) пациентов ее эффект оценен как стабилизация опухолевого процесса, у 5 (56%) зарегистрирован частичный регресс.
В этой группе среднее число введений составило 7,1 (3–16), среднее время наблюдения после окончания лечения – 8,5 (3,0–14,2) месяца.
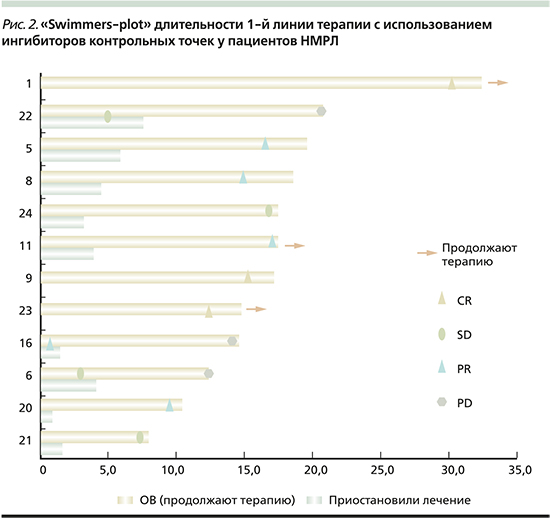
Прогрессирование после досрочного окончания терапии зафиксировано у 3 пациентов: PD-L1>/=50–1/3, PD-L1 1–49% – 1/3, PD-L1 0% – 1/3 мВДП составило 9,3 (7,1–13,2) месяца, 6 (67%) наблюдаются без прогрессирования 9,3 (4,0–12,1) месяца (мин-макс). За период наблюдения медиана ВБП не была достигнута. Распределение по эффектам и длительности терапии среди пациентов наглядно продемонстрировано на рис. 2.
Обсуждение
В нашей работе была оценена эффективность сокращенного из-за непреодолимых организационных препятствий курса ИТ неоперабельного рака легкого в реальной клинической практике. В результате наблюдения за больными, получавшими монотерапию ИИКТ в качестве 1-й линии терапии в среднем около 7 циклов и достигшими клинического эффекта, прогрессирование в течение в среднем 8,5 месяцев наблюдения без введения лекарственных препаратов развилось у 3 из 9 пациентов.
На сегодняшний день единственное исследование, в котором проведен анализ эффективности сокращенного цикла ИТ при неоперабельном раке легкого, – это CheckMate 153 [8]. В данном исследовании изучалась эффективность фиксированной длительности терапии на протяжении года по сравнению со второй группой пациентов, получавших терапию до регистрации прогрессирования или непереносимой токсичности. Необходимо отметить, что данное исследование проводилось в группе пациентов, получавших 2-ю линию системной терапии. Его результаты продемонстрировали более высокие показатели выживаемости в группе пациентов, кому лечение ИТ было продолжено до регистрации прогрессирования. Безусловно, результаты этого исследования нельзя в полной мере экстраполировать на группу пациентов с высоким уровнем PD-L-экспрессии, получавших терапию ИИКТ точек в рамках 1-й линии системной лекарственнойтерапии.
Заключение
Таким образом, сохраняется необходимость проведения новых исследований с целью изучения оптимальной длительности ИТ.
Полученные нами данные косвенно свидетельствуют об отсутствии тенденции к уменьшению мВБП и мОВ в группе пациентов с НМРЛ, получавших мИТ в 1-й линии лечения, кому внепланово было прекращено лечение, а не продолжено до 2 лет или до регистрации прогрессирования опухолевого процесса.



