Введение
По данным GLOBOCAN, в 2020 г. в мире зарегистрировано 430 тыс. новых случаев заболевания почечно-клеточным раком (ПКР), умерли около 180 тыс. пациентов с данным диагнозом [1]. Не менее 30% больных с впервые выявленным раком почки имеют распространенную или метастатическую форму заболевания с преимущественной локализацией метастазов в легких и костях. Результаты фундаментальных исследований, проведенных за последние десятилетия в области биологии ПКР, сформировали современные представления о принципиальных механизмах патогенеза опухоли, а также о потенциальных терапевтических возможностях. В настоящее время основной лечебной тактикой в отношении метастатического ПКР (мПКР) является системная лекарственная терапия.
Рак почки, возникающий из клеток проксимальных извитых канальцев, в 75–85% случаев имеет светлоклеточный гистологический подтип, который характеризуется специфическими альтерациями в гене Von Hippel Lindau (VHL). Инактивация гена сопровождается усилением ангиогенеза, в т.ч. повышением активности факторов ангиогенеза, таких как сосудистоэндотелиальный фактор роста (VEGF). Современные ингибиторы тирозинкиназ (ИТК) VEFG (пазопаниб, сунитиниб, акситиниб и др.) успешно воздействуют на данный патогенетический механизм, угнетая его [2].
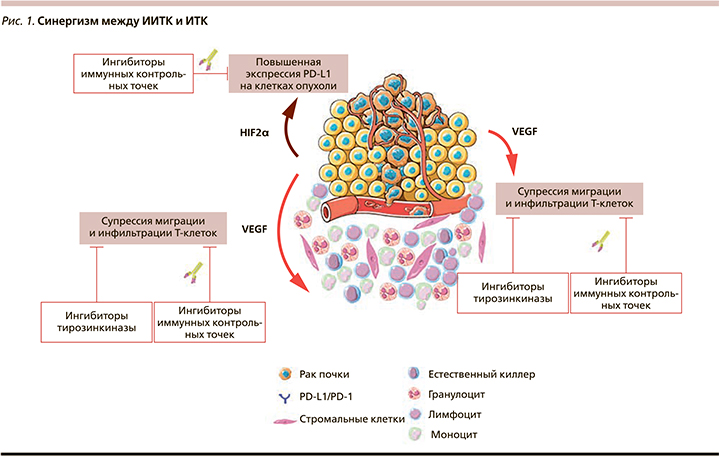
Одной из важнейших характеристик ПКР является его иммуногенность, выявленная более полувека назад и подтвержденная уже в XXI в. результатами крупных рандомизированных исследований. В настоящее время не вызывает сомнений наличие выраженного взаимодействия между иммунной системой и ангиогенезом, в т.ч. и патологическим. Так, проангиогенные факторы обладают способностью подавлять дифференцировку, созревание и миграцию Т-лимфоцитов в микроокружение опухоли, а также изменять функциональный статус PD-L1 на поверхности опухолевых клеток (рис. 1). Биологические предпосылки к одновременному воздействию на компоненты иммунной системы, эндотелий сосудов опухоли и ее микроокружение лежат в основе комбинированного применения ИТК и ингибиторов иммунных точек контроля (ИИТК) в отношении больных распространенным и метастатическим раком почки [2, 3].
До настоящего времени как в многоцентровых рандомизированных исследованиях, так и в рутинной клинической практике применяются две базовые прогностические шкалы (MSKCC и IMDC), разработанные в эру интенсивного изучения ИТК с целью стратификации больных на основании наличия или отсутствия ряда биологических характеристик пациента и опухоли [4]. Недостатком данных прогностических моделей в эпоху изучения и применения регуляторов контрольных точек иммунного ответа является их неспособность предсказать реализацию эффекта иммунотерапии. Такие биологические маркеры, как уровень экспрессии PD-L1 и VEGF, а также мутационная нагрузка, не продемонстрировали убедительной предиктивной значимости при применении ИИТК у больных ПКР. Возможно предположить существование определенных биологических или молекулярных кластеров, формирующих т.н. ангиогенный или иммуногенный фенотип ПКР и способных предсказывать чувствительность опухоли к ингибиторам ангиогенеза или контрольных точек иммунного ответа [5, 15, 16]. Так, в исследовании II фазы IMmotion 150 сунитиниб успешно реализовывал свою эффективность в отношении опухолей почки с высокой экспрессией генов ангиогенеза, в то время как их малая экспрессия ассоциировалась с большей эффективностью комбинации атезолизумаба и бевацизумаба. По-видимому, выявление определенных генетических сигнатур и их включение в модифицированные прогностические шкалы могут иметь значение в выборе оптимального варинта системной терапии ПКР [6].
На протяжении последних 5 лет исследователи представляли результаты пяти новых комбинаций с включением ингибиторов ангиогенеза и иммунных точек контроля у больных распространенным и метастатическим раком почки. В данном обзоре более подробно рассмотрены результаты многоцентрового рандомизированного исследования III фазы JAVELIN Renal 101, в котором изучалась эффективность комбинации авелумаба и акситиниба [7]. Авелумаб, человеческое моноклональное антитело против PD-L1, обладает противоопухолевой активностью в отношении ряда солидных опухолей, включая ПКР. Акситиниб, высокоселективный ИТК рецепторов VEGF-1, VEGF-2 и VEGF-3, обеспечивает мощное ингибирование пролиферации и выживаемости клеток эндотелия опухоли. Препарат продемонстрировал доказанную эффективность и приемлемую токсичность в качестве первой и второй линий лекарственной терапии мПКР. Значимая противоопухолевая активность каждого из препаратов стала весомым основанием для изучения их синергизма в доклинических, а в последующем и в клинических исследованиях [8].
Результаты рандомизированного исследования III фазы JAVELIN Renal 101
В период с 29.03.2016 по 19.12.2017 в рамках исследования JAVELIN Renal 101 были рандомизированы 886 больных распространенным ПКР с преимущественным светлоклеточным компонентом опухоли, ранее не получавших лекарственной терапии. К основным критериям включения относились наличие не менее одного измеряемого очага (в соответствии с критериями RECIST версии 1.1) и общесоматический статус 0–1 (в соответствии со шкалой ECOG). По результатам рандомизации 442 пациента были распределены в группу комбинации авелумаба и акситиниба, 444 – в группу монотерапии сунитинибом. Из 886 больных 560 (63,2%) имели опухоли с положительной экспрессией PD-L1 (270 и 290 в группах комбинации и сунитиниба соответственно).
Первичными конечными точками в исследовании служили выживаемость без прогрессирования (ВБП) и общая выживаемость (ОВ) больных ПКР, имевших позитивную экспрессию PD-L1 в опухоли (PD-L1≥1%). В качестве вторичных конечных точек изучались ВБП и ОВ у больных общей популяции, а также частота объективных ответов (ЧОО) и их длительность (по оценке BICR и исследователей).
Авелумаб назначался в дозе 10 мг/кг массы тела в виде 60-минутной инфузии каждые 2 недели с премедикацией антигистаминными и антипиретическими средствами. Акситиниб применялся перорально, непрерывно в начальной дозе 5 мг 2 раза в сутки, при этом была возможна коррекция суточной дозы препарата (повышение и снижение). В контрольной группе пациенты принимали сунитиниб перорально, в стандартной дозе – 50 мг в сутки в течение 28 дней каждые 6 недель. Согласно инструкции по применению препарата, производилось редуцирование его дозы при развитии токсичности.
Первый промежуточный анализ результатов исследования JAVELIN Renal 101 произведен при достижении минимального периода наблюдения, равного 6 месяцам. Комбинация авелумаба и акситиниба продемонстрировала значительное увеличение ВБП по сравнению с сунитинибом у больных с PD-L1+ПКР (ОР=0,61; 95% ДИ: 0,47–0,79; p≤0,001), а также в общей популяции больных (ОР=0,69; 95% ДИ: 0,56–0,84; p≤0,001). Медиана ОВ на тот период не была достигнута.
На момент среза данных в рамках второго промежуточного анализа (28.01.2019, минимальный период наблюдения – 13 месяцев) 242 (54,8%) пациента закончили терапию как авелумабом, так и акситинибом; 336 (75,7%) завершили прием сунитиниба. Основной причиной прекращения лечения было прогрессирование ПКР. Продолжили прием авелумаба и акситиниба 170 (38,5%) больных, только авелумаба – 8 (1,8%), только акситиниба – 22 (5,0%). 108 (24,3%) пациентов продолжали терапию сунитинибом.
Больным PD-L1+распространенным раком почки комбинированная терапия авелумабом и акситинибом обеспечила значительное улучшение ВБП по сравнению с контрольной группой (ОР=0,62; 95% ДИ: 0,490–0,777; p≤0,0001). Медианы ВБП составили 13,8 и 7,2 месяца в изучаемой и контрольной группах соответственно. Сходные результаты продемонстрированы в общей популяции больных (ОР=0,69; 95% ДИ: 0,574–0,825; p≤0,0001) (рис. 2).

Медиана ОВ не была достигнута. В популяции PD-L1+больных ОР составил 0,83 (95% ДИ: 0,596–1,151; p=0,1301). Летальный исход от любой причины зарегистрирован у 66 (24,4%) больных в группе комбинированной терапии и у 79 (27,2%) в группе сунитиниба. ОР в общей популяции составил 0,80 (95% ДИ: 0,616–1,027; p=0,0392).
Последующая противоопухолевая терапия в группе комбинации авелумаба и акситиниба проведена значительно меньшему числу больных (138 [31,2%]) по сравнению с группой сунитиниба (227 [51,1%]). Любые ИИТК назначены лишь 33 (7,5%) больным группы комбинированной терапии по сравнению с 159 (35,8%) контрольной группы. В целом на основании анализа RPSFT с учетом возможного применения любого ИИТК в группе сунитиниба предполагается уменьшение риска смерти на 35% как в общей (ОР=0,65, 95% ДИ: 0,413 0,933), так и в PD-L1+популяции больных (ОР=0,65; 95% ДИ: 0,337–1,050).
ЧОО в общей популяции больных составила 52,5% (95% ДИ: 47,7–57,2) (полный регресс в 3,8% случаев) в группе комбинации авелумаба и акситиниба по сравнению с 27,3% (95% ДИ: 23,2–31,6) (полный регресс – 2,0%) в группе сунитиниба. Аналогичные результаты с практически двукратным увеличением ЧОО, в т.ч. полных ответов от экспериментальной группы, получены и от популяции PD-L1+ пациентов (табл. 1).

В общей популяции больных, ответивших на лечение комбинацией авелумаба и акситиниба, эффект регистрировался значительно раньше по сравнению с группой контроля. Медианы времени до наступления ответа составили 2,7 (1,2–20,7) и 4,0 месяца (1,2–18,0) соответственно. Объективные ответы в обеих группах характеризовались длительностью и устойчивостью. В общей популяции медиана длительности ответа составила 18,5 месяца (95% ДИ: 17,8–не поддающийся оценке) в группе комбинированной терапии и не была достигнута в группе сунитиниба (95% ДИ: 16,4–не поддающийся оценке). В популяции PD-L1+больных медиана длительности ответа также составила 18,5 месяца (95% ДИ: 17,8–не поддающийся оценке) в группе комбинации и не была достигнута в группе сунитиниба (95% ДИ: 11,2–не поддающийся оценке). Средняя длительность ответа в данной популяции была на 4,7 месяца больше в группе комбинации авелумаба и акситиниба по сравнению с группой контроля (95% ДИ: 3,1–6,3). Не вызывает сомнений значение выбора оптимальной лекарственной терапии первой линии в отношении эффективности второй и последующих линий. С целью оценки влияния обоих вариантов лечения на показатель ВБП при проведении второй линии терапии (ВБП2) в рамках исследования JAVELIN Renal 101 проведен дополнительный анализ. Комбинация авелумаба и акситиниба продемонстрировала преимущества по сравнению с сунитинибом как в общей (ОР=0,55 (95% ДИ: 0,440–0,688)), так и в PD-L1+ (ОР=0,52 [95% ДИ: 0,395 0,694]) популяциях больных.
Важно отметить, что применение комбинированной терапии обеспечило преимущество перед монотерапией сунитинибом по показателям ВБП, ОВ и ЧОО во всех прогностических группах (MSKCC, IMDC) вне зависимости от общесоматического статуса (ECOG PS) и статуса PD-L1 (табл. 2).

Один из принципиальных выводов, которые мы можем сформулировать исходя из результатов клинического исследования JAVELIN Renal 101, – это малая эффективность монотерапии сунитинибом для больных с повышенной экспрессией PD-L1 в опухоли. Как уже указывалось выше, медиана ВБП в группе больных, получавших сунитиниб, составила лишь 7,2 месяца, на 6,6 месяца уступив комбинации авелумаба и акситиниба [7, 9].
Профиль токсичности обоих вариантов лекарственной терапии представлен типичными для ИТК и ИИТК нежелательными явлениями. Частота осложнений всех степеней, а также 3-й степени и выше в группе комбинации авелумаба и акситиниба составила 99,5 и 71,2%, в группе сунитиниба – 99,3 и 71,5% соответственно. Из 434 больных, получавших комбинированное лечение, 166 (38,2%) имели иммуноопосредованные нежелательные явления, наиболее частыми из которых были эндокринопатии (24,7%). В целом токсичность лекарственной терапии в обеих исследуемых группах характеризовалась приемлемостью и управляемостью [10, 11].
Таким образом, комбинация авелумаба и акситиниба обладает достоверным преимуществом по сравнению с сунитинибом в качестве терапии первой линии больных мПКР с преимущественным светлоклеточным компонентом, которое реализуется прежде всего в PD-L1+популяции в виде значительного (на 38%) снижения риска прогрессирования заболевания. Кроме того, во всех подгруппах больных монотерапия сунитинибом уступает по показателям ВБП, ЧОО, длительности ответа и ВБП2 [7, 9, 17].
Безусловный интерес представляют попытки определения места рассматриваемой комбинации среди других известных к настоящему времени режимов комбинированной терапии первой линии мПКР. Помимо комбинации авелумаба и акситиниба в современный арсенал лекарственного лечения распространенного рака почки входят комбинации пембролизумаба и акситиниба, пембролизумаба и ленватиниба, ниволумаба и кабозантиниба, ниволумаба и ипилимумаба, а также атезолизумаба и бевацизумаба. Отсутствие прямых («head-to-head») сравнений данных режимов в рамках рандомизированных исследований побуждает исследователей к проведению метаанализов. В 2022 г. N.A. Bosma et al. представлены результаты мета-анализа шести многоцентровых рандомизированных клинических исследований III фазы с участием 5121 больного распространенным ПКР. На основании проанализированных данных исследователи предполагают, что комбинация авелумаба и акситиниба (наряду с комбинацией ниволумаба и кабозантиниба) демонстрирует преимущество в отношении ЧОО и ОВ в популяции больных с благоприятным прогнозом, а также (наряду с комбинациями ниволумаба и кабозантиниба, пембролизумаба и акситиниба и пембролизумаба и ленватиниба) в отношении ВБП по сравнению с сунитинибом [6].
Саркоматоидный рак почки
Лекарственная терапия распространенного саркоматоидного рака почки по-прежнему остается нерешенной проблемой современной клинической онкологии. Саркоматоидный ПКР (сПКР) относится к редким гистологическим подтипам, составляя не более 5–8% от всех случаев рака почки. В то же время сПКР обладает агрессивным фенотипом, определяющим наихудший прогноз для жизни больных. Большинство пациентов, страдающих саркоматоидным раком почки, во время установления диагноза имеют распространенную или метастатическую стадию заболевания. Биологической особенностью сПКР является наличие выраженной инфильтрации опухоли иммунными клетками (в частности, регуляторными Т-лимфоцитами, активированными натуральными киллерами, клетками памяти и дендритными клетками, М1и М2-макрофагами) наряду с высокой экспрессией PD-(L)1 на их поверхности. По мнению большинства исследователей, сПКР относится к т.н. «горячим» опухолям с иммуновоспалительным микроокружением, что безусловно позволяет ожидать реализации эффекта ИИТК [12, 13].
В рамках клинического исследования JAVELIN Renal 101 помимо больных светлоклеточным ПКР принимали участие 108 пациентов с саркоматоидным компонентом опухоли (47 из группы комбинации авелумаба и акситиниба, 61 из группы сунитиниба). Данные подгруппы были хорошо сбалансированы и соответствовали характеристикам основной популяции. Большинство больных имели промежуточный или плохой прогноз (в соответствии с критериями MSKCC) (табл. 3).

Применение комбинации авелумаба и акситиниба у больных сПКР привело к значимому увеличению ВБП по сравнению с сунитинибом (ОР=0,57 [95% ДИ: 0,325–1,003]). Медианы ВБП составили 7,0 (95% ДИ: 5,3–13,8 месяца) и 4,0 месяца (95% ДИ: 2,7–5,7 месяца) соответственно. Летальный исход от любой причины зарегистрирован у 11 больных (23,4%) группы комбинированной терапии и у 20 (32,8%) контрольной группы. ОВ через 12 месяцев составила 83% (95% ДИ: 67,3–91,6%) и 67% (95% ДИ: 51,9–78,3%) в обеих группах.
Применение комбинации авелумаба и акситиниба обеспечило достижение частичного регресса 20 (42,6%) и полного регресса 2 (4,3%) из 47 больных сПКР. В группе сунитиниба лишь у 13 пациентов из 61 (21,3%) зарегистрирован частичный регресс. Таким образом, ЧОО составила 46,8% (95% ДИ: 32,1–61,%) против 21,3% (95% ДИ: 11,9–3,7%) в исследуемой и контрольной группах соответственно. Медиана времени до регистрации объективного ответа составила 1,6 (1,2–9,8 месяца) в группе комбинации авелумаба и акситиниба по сравнению с 3,1 (1,2–11,1 месяца) в группе сунитиниба [14]. Полученные в клиническом исследовании JAVELIN Renal 101 данные, свидетельствующие о преимуществах комбинации авелумаба и акситиниба перед монотерапией сунитинибом в терапии первой линии больных сПКР, коррелируют с результатами аналогичных поданализов, проведенных в рамках других рандомизированных исследований III фазы по изучению эффективности комбинаций пембролизумаба и акситиниба (KEYNOTE-426), ниволумаба и ипилимумаба (CheckMate 214) и атезолизумаба и бевацизумаба (IMmotion 151).
Заключение
Современный арсенал лекарственного лечения распространенного рака почки пополнился новой комбинацией ИИТК и ИТК, доказавшей высокую эффективность в рамках рандомизированного исследования III фазы. Биологически обоснованный синергизм авелумаба и акситиниба реализуется значительным улучшением показателей выживаемости без прогрессирования и увеличением частоты и длительности объективных ответов от больных светлоклеточным мПКР независимо от уровня экспрессии PD-L1 и прогностической группы риска. Применение комбинации авелумаба и акситиниба у больных раком почки с саркоматоидным компонентом демонстрирует отчетливое превосходство по сравнению сунитинибом, расширяя возможности эффективного лечения этой прогностически неблагоприятной когорты пациентов.
Финансирование. Отсутствует.


