Введение
Кистозный фиброз поджелудочной железы является типичной генетической экзокринопатией, в основе которой лежит мутация гена, контролирующего структуру и функцию трансмембранного регулятора проводимости муковисцидоза (cystic fibrosis transmembrane regulator – CFTR). Генетическая вариабельность гена CFTR может быть ассоциирована как со сниженным количеством, так и с полным отсутствием трансмембранного белка, который обеспечивает транспорт электролитов, а именно ионов хлора и натрия, в клетках экзокринных желез, что приводит к нарушению электрического потенциала клетки, дегидратации секрета, изменению его вязкости [1, 2].
До недавнего времени терапия больных муковисцидозом носила исключительно симптоматический характер, включая местные и системные антибактериальные препараты, муколитики различных классов, в т.ч. гиперосмолярные растворы, ферментотерапию, комплексы кинезиотерапии, ориентированные на возраст и состояние пациентов. Таким образом, комплекс лечебных мероприятий, с одной стороны, был ориентирован на разрыв замкнутого круга «воспаление—инфекция—воспаление», что приводило к улучшению клиренса бронхиального дерева и контролю легочных инфекций, с другой, заместительная терапия микросферическими ферментами с высокой липазной активностью компенсировала недостаточность поджелудочной железы, улучшая нутритивный статус больных. Такой комплекс мероприятий обеспечивал определенный контроль над течением заболевания, способствуя определенному повышению качества и продолжительности жизни больных с кистофиброзом поджелудочной железы. Новые достижения науки и фармацевтической промышленности развернули перед больными муковисцидозом новые перспективы. Несмотря на то что попытки создания этиотропного препарата (Аталурен), способного вызывать остановку стоп-кодона РНК-полимеразой с транскрипцией гена, оказались неудачными в связи с низкой клинической эффективностью, разработки были продолжены, но с переносом фокуса внимания на внутриплазматическую модификацию уже синтезированного белка и потенцирование его функции при встраивании в апикальную мембрану. В настоящее время все препараты таргетной терапии муковисцидоза подразделяются на два класса: корректоры, непосредственно способствуюющие образованию 3D-структуры трансмембранного белка, и потенциаторы, модифицирующие функцию встроенного в апикальную мембрану хлорного канала. Многообразие генетических мутаций и ассоциированных с ними вариантов структурной несостоятельности и дисфункции белка CFTR диктует дифференцированный подход к выбору патогенетической терапии. Для некоторых вариантов изменения генетической последовательности, относящихся к III–IV классам, рекомендован потенциатор ивакафтор, разрешенный к использованию у младенцев. В качестве патогенетической терапии для больных с генетическим диагнозом F508del/F508del предназначена комбинация корректора лумакафтора и потенциатора ивакафтора (Оркамби), а в случае гетерозиготного носительства мутации класса F508del с одним из 177 генетических вариантов с минимальной функциональностью используется тройная комбинация: ивакафтор/тезакафтор/элексакафтор (Трикафта). Комбинированные препараты способны оказывать двойное действие, за счет чего регулируется транспорт электролитов между клетками и внеклеточной жидкостью, что приводит к нормализации вязкости секрета экзокринных желез [1, 2].
В Российской Федерации таргетная терапия для пациентов с кистофиброзом поджелудочной железы стала доступной с начала 2021 г., когда в нашей стране был зарегистрирован препарат ивакафтор/лумакафтор (Оркамби). Безусловно, появление таргетной терапии для больных муковисцидозом открывает новые перспективы в отношении продолжительности и качества жизни. В настоящей статье описан опыт 12-недельного применения ивакафтора/лумакафтора у троих детей с муковисцидозом в Астраханской области.
Методы
Основу клинического исследования составили трое пациентов мужского пола с тяжелым течением муковисцидоза в возрасте 6, 11 и 14 лет. Диагноз больным был выставлен на 4—5-й неделе жизни по результатам совокупной оценки критериев заболевания в диагностических блоках: сочетание клинических проявлений (респираторного и кишечного синдромов), неонатального скрининга с положительными показателями потового теста и/или определение мутантных фенотипасоциированных аллелей в гене CFTR. Пациенты проходили регулярное комплексное обследование и лечение на базе ГБУЗ АО ОДКБ им. Н.Н. Силищевой в Астрахани.
Базисная терапия, согласно национальным рекомендациям, включала муколитические препараты (представленные дорназой-альфа и гипертоническим раствором NaCl), микросферические ферменты с высокой липазной активностью, гепатопротекторы, витаминотерапию. Для эффективной эвакуации мокроты медикаментозное лечение сопровождалось кинезиотерапией, ориентированной на возраст и состояние пациентов. С учетом идентификации в микробиоте дыхательных путей Pseudomonas aeruginosa проводились курсы эрадикационной терапии ингаляционными аминогликазидами. Среди осложнений муковисцидоза у наблюдаемых пациентов были выделены следующие: у пациента К. (20.07.2007 г. р.) – полипоз носа, цирроз печени, отставание в росте (SD роста – 2); пациент Ш. (19.04.2011 г. р.) – полипоз носа, белково-энергетическая недостаточность (SD ИМТ – 2); пациент С. (01.08.2015 г. р.) – полипы в носовых ходах, цирроз печени, белково-энергетическая недостаточность (SD ИМТ – 3). Состояние исследуемых детей расценивалось как среднетяжелое, обострения бронхолегочного процесса не было диагностировано.
Критерии для проведения таргетной терапии ивакафтором/лумакафтором: 1) мутация F508del в гомозиготном положении; 2) возраст >6 лет; 3) наличие грамотрицательной флоры в микробиоте дыхательных путей; 4) показатели функции внешнего дыхания (ФВД) менее 80%. Пациентам до начала лечения ивакафтором/лумакафтором дополнительно проведена ДНК-диагностика для исключения гомизиготности и носительства варианта L467F гена CFTR в составе комплексного аллеля с мутацией F508del.
Согласно рекомендациям по таргетной терапии ивакафтором/лумакафтором, предстартовый диагностический протокол включал определение антропометрических данных (рост, вес, ИМТ); биохимического анализа крови с уточнением показателей печеночных трансаминаз и общего биллирубина, уровня панкреатической эластазы; измерение хлоридов потовой жидкости; оценку ФВД (по показателям ОФВ1, ФЖЕЛ, индекс Тиффно), динамическое измерение артериального давления. Мониторирование вышеперечисленных показателей производилось в декретированные сроки: на 14-е сутки, через 4 и 12 недель от начала терапии. Для исключения патологии глазного дна, исключения формирования катаракты проводилась консультация офтальмолога.
Согласно рекомендациям производителя, взависимостиотвозрастаисследуемый препарат назначался в следующих дозировках: по 2 таблетки ивакафтора 125 мг и лумакафтора 200 мг 2 раза в день для пациента 14 лет; по 2 таблетки ивакафтора 125 мг и лумакафтора 100 мг 2 раза в день для пациентов младшей группы – 10 и 6 лет.
Результаты
На рис. 1–3 приведена динамика показателей по ключевым точкам у пациентов, получавших таргетную терапию ивакафтором/лумакафтором в возрастных дозировках.
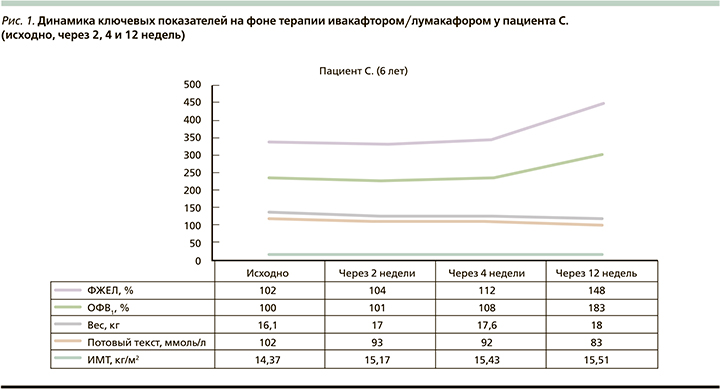
У пациента С. (рис. 1) за время терапии отмечалась положительная динамика по основным ключевым точкам: увеличение масса-ростовых показателей (ИМТ+1,14 кг/м2), прирост показателей ФВД на фоне снижения показателей уровня хлоридов в потовой жидкости. Клинически было отмечено уменьшение респираторных симптомов (кратность и интенсивность кашля), улучшение аппетита и общего самочувствия ребенка в виде повышения дневной активности. При ежемесячном мониторировании дыхательной микрофлоры в течение 3 месяцев роста Pseudomonas aeruginosa не наблюдалось. Необходимо отметить, что,несмотря на курсы внутривенной и ингаляционной терапии, проводимой в течениие 3 лет ранее, добиться эрадикации грамотрицательной флоры не удавалось.
Несмотря на то что степень панкреатической недостаточности продолжала носить тяжелый характер, наблюдалось повышение уровня эластазы с 15 до 78 мкг/г. На 5-й день приема ивакафтора/ лумакафтора были зарегистрированы нежелательные побочные явления в виде болей в эпигастральной области, которые купировались самостоятельно и не повлекли отмены препарата.
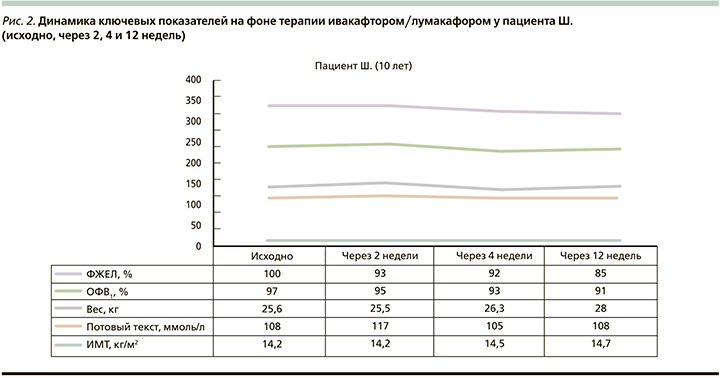
За время наблюдения у пациента Ш. (рис. 2) наблюдалось увеличение масса-ростовых показателей (рост+3 см, вес+2,4 кг, ИМТ+0,5 кг/м2), улучшение аппетита, общего самочувствия. Однако уровень хлоридов потовой жидкости и ключевых показателей ФВД (ОФВ1 и ФЖЕЛ) оставались без динамики, что ставит под сомнение эффективность проводимой таргетной терапии для данного пациента.
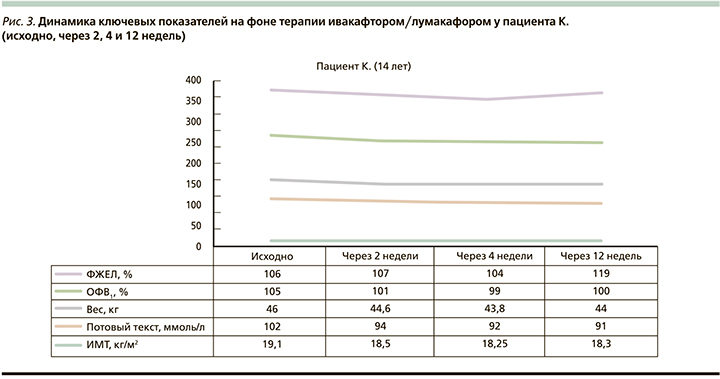
Согласно результатам, представленным на рис. 3, респираторная функция характеризовалась достоверным при ростом показателя ФЖЕЛ, который составил 13% для относительных значений и 0,9 л для абсолютных. Уровень хлоридов пота снизился на 8 единиц в первые 14 дней приема препарата, в дальнейшем данный показатель сохранялся без существенной динамики. В ходе исследования у данного больного наблюдался регресс масса-ростовых значений, а именно отмечен уверенный тренд снижения ИМТ с 19,1 до 18,3 кг/м2 в течение первых 4 недель приема Оркамби. Пациент отмечал ухудшение общего самочувствия, появление слабости, повышенной утомляемости, а на первой неделе приема испытуемого препарата – диспепсические проявления и болевой абдоминальный синдром. На 12-й неделе наблюдения было зарегистрировано серьезное нежелательное явление в виде нарастания уровня печеночных трансаминаз (уровень АЛТ на старте терапии – 73,5 ЕД/л, через 12 недель – 224,8 ЕД/л; уровень АСТ – 50,8 и 247,6 ЕД/л соответственно). Несмотря на попытку стабилизации биохимических показателей путем отмены с последующим возобновлением приема ивакафтора/лумакафтора в дозе, составившей половину от терапевтической, наблюдалось повторное нарастание цитолитической активности печени, что повлекло за собой полную отмену препарата.
Обсуждение
Эффективность и безопасность комбинированного препарата ивакафтор/ лумакафтор была оценена в клинических исследованиях с участием более 1100 пациентов в возрасте старше 12 лет и более 320 пациентов младше 12 лет, в т.ч. и детей до 6 лет.
Согласно результатам исследований, детям старше 12 лет удалось достичь статистически значимого (р<0,001) повышения показателей ФВД (увеличение ОФВ1 в абсолютном исчислении на 3,3–2,8%) и ИМТ (на 0,24–0,28 кг/м2), при этом наблюдалось значительное снижение частоты бронхолегочных обострений. За время наблюдения отмечена хорошая переносимость комбинированного препарата, однако у 13% детей отмечались нежелательные явления на фоне терапии в виде инфекционных обострений бронхолегочного процесса, кровохарканья, а также умеренного повышения артериального давления [3–6].
Не менее значимые результаты по эффективности и безопасности применения ивакафтора/лумакафтора в отношении детей 6–12 лет получены в рандомизированном плацебо-контролируемом исследовании в параллельных группах фазы III [7, 8] и детей до 6 лет в исследованиях VX15-809-115 и VX16-809-116, которые продолжались в течение 120 недель [9]. На фоне терапии отмечены улучшение показателей ФВД, снижение концентрации хлоридов в потовой жидкости на 29,6 ммоль/л от исходного, восстановление функции поджелудочной железы в виде увеличения концентрации эластазы до 200 мкг/г и более на 96-й неделе исследования VX16-809-116, а также прибавка в росте [9].
Результаты, полученные в нашем исследовании пациента С., сопоставимы с этими данными. У больного отмечен хороший ответ на терапию ивакафтором/лумакафтором по ключевым точкам в виде прироста ФВД, увеличения ИМТ, снижения уровня хлоридов в потовой жидкости, при этом не было зафиксировано нежелательных явлений, повлекших за собой отмену терапии. Возможно, причиной столь выраженного прироста основных показателей функции внешнего дыхания (ОФВ1 на 0,74 л и ФЖЕЛ на 0,97 л) у пациента стала не только нормализация работы хлорного канала, но и эрадикация Pseudomonas aeruginosa в микробиоте дыхательного тракта, которую удалось достичь впервые за 3 года с момента первичного высева.
Согласно результатам исследования VX14-809-109, у 13% детей зарегистрированы различного рода нежелательные явления, повлекшие прекращение таргентной терапии 3% пациентов [7, 8]. Ведущими стали нежелательные явления, ассоциируемые с развитием обострений бронхолегочного процесса и повышения уровня печеночных трансаминаз относительно референтных значений. Согласно результатам нашего исследования, у одного больного (пациент К.) на фоне терапии ивакафтором/лумакафтором наблюдалось повышение цитолитической активности печени, повлекшее отмену препарата на 12-й неделе терапии. Доказано, что ивакафтор подвергается метаболизму в печени через систему цитохрома P-450/CYР3A. По нашему мнению, цитотоксический эффект, наблюдаемый у нашего пациента, произошел под действием токсичных или иммуногенных продуктов распада ивакафтора, на фоне сниженной метаболической активности печени, ассоциированной с циррозом, у пациента К. С другой стороны, возможно, больной является носителем вариантов генетических последовательностей гена CYP2D6, обеспечивающих замедление биотрансформации лекарственных веществ с реализацией нежелательных реакций. В связи с этим планируется генетическое исследование методом полимеразной цепной реакции для обнаружения вариантов полиморфизма гена цитохрома P-450/CYР3A с целью выбора режима дозирования препарата.
У пациента Ш., несмотря на положительную клиническую динамику, не прослеживалось позитивных изменений по основным контрольным точкам исследования (уровень хлоридов потовой жидкости и показатели ФВД), что может быть связано, с одной стороны, с коротким периодом наблюдения, с другой – с наличием других комплексных аллелей помимо полиморфизма L467F гена CFTR, которые также могут приводить к рефрактерности терапии ивакафтором/лумакафтором. Согласно протоколу проведения таргетной терапии, решение вопроса о целесообразности дальнейшего использования препарата Оркамби будет принято по истечении 6 месяцев приема после проведения комплексного клиниколабораторного обследования. В случае отсутствия положительной динамики будет проведено генотипирование CFTR с целью выявления дополнительных комплексных аллелей, отличных от L467F, и решение вопроса о пересмотре препарата таргетной терапии.
Заключение
За 12 недель исследования получены неоднозначные результаты терапии ивакафтором/лумакафтором. С одной стороны, открываются благоприятные перспективы улучшения течения и прогноза заболевания за счет нормализации работы хлорного канала и улучшения клинического статуса пациента, с другой – развитие серьезных нежелательных явлений может проводить к отказу от выбранной терапии. Все вышеизложенное указывает на необходимость увеличения длительности наблюдения с вовлечением в исследование новых пациентов для объективизации и оценки долгосрочных результатов.
Финансирование. Исследование не имело спонсорской поддержки.



