Введение
Вилочковая железа (ВЖ) достигает максимальной массы в период полового созревания, после чего с возрастом происходят инволютивные изменения в виде сокращения плотной и корковой ткани, что снижает тимопоэтическую продуктивность. У лиц старше 40 лет ВЖ обычно представлена жировой тканью, но выработка Т-клеток сохраняется [1]. Наряду с этим острые стрессовые факторы, такие как химиотерапия (ХТ), радиотерапия, операции, острые воспалительные заболевания, трансплантация стволовых клеток, ожоговые повреждения кожи, могут вызывать структурную перестройку ткани ВЖ и нарушения функциональной активности. По данным P.L. Choyke (1987), гипоплазия ВЖ наблюдается более чем у 90% молодых людей в возрастном диапазоне 2–35 лет, средний возраст – 17 лет, получающих ХТ по поводу злокачественных образований [2].
Так, Dao-Ping Sun et al. (2016) показали значительное снижение в периферической крови уровня CD4+-клеток и Т-регуляторных лимфоцитов у 54 пациентов с В-клеточной лимфомой по окончании ХТ. Эти показатели восстанавливались до исходного уровня и выше через 9 месяцев по завершении полихимиотерапии [3].
После прекращения токсического воздействия противоопухолевой терапии у 10–25% больных происходит диффузное увеличение: гиперплазия тимуса без изменения формы и гистологического строения [4].
Впервые гиперплазия тимуса после ХТ была описана в 1983 г. и представляет, вероятно, иммунологический феномен, который может свидетельствовать о восстановлении иммунитета [5].
В литературе есть сообщения, что у пациентов с развивающейся гиперплазией ВЖ после ХТ происходит более быстрое восстановление функции тимуса [6, 7].
Частота встречаемости гиперплазии ВЖ зависит прежде всего от возраста. Так, у детей и подростков гиперплазия тимуса наблюдается в 25% случаев, что подтверждает высокую пластичность тимуса у этих пациентов [2, 8, 9].
Феномен гиперплазии тимуса у взрослых описан редко [10].
В исследовании Dao-Ping Sun et al. (2016) наблюдали гипоплазию тимуса у 30% взрослых (при медиане возраста 35 лет), а гиперплазия отмечалась в 37% случаев, в то время как у лиц старше 60 лет она не встречалась [3].
Другие авторы отмечали гиперплазию тимуса по окончании лечения у 63% взрослых пациентов, средний возраст – 30 лет, при диапазоне 18–49 лет и в то же время отсутствие ее у пожилых исследуемых. Они считают, что ВЖ способна к регенерации по крайней мере до среднего возраста, внося существенный вклад в восстановление периферических Т-лимфоцитов после ХТ [11].
Реактивная гиперплазия тимуса после ХТ у больных лимфомами сопровождается диффузным увеличением жировой и лимфоидной ткани железы, которая ошибочно может быть принята за рецидив или остаточную опухоль. Такая ситуация сопровождается выполнением необоснованных диагностических и лечебных опций, включая тимэктомию [3, 12, 13].
Для дифференциальной диагностики образований в средостении применяют рентгеновскую компьютерную, магнитно-резонансную томографии и гибридные технологии – ПЭТ/КТ [13, 14].
Bala Basak Oven Ustaalioglu et al. (2011) приводят результаты ретроспективного анализа компьютерных томограмм у 140 пациентов с лимфомой Ходжкина (ЛХ) и неходжкинскими лимфомами. Всем больным проведена комбинированная химиотерапия по схеме ABVD при ЛХ и R-CHOP при неходжкинских лимфомах. В 5 (3,6%) случаях (ЛХ – 4, неходжкинская лимфома – 1 пациент) по окончании лечения и достижения полной клинической ремиссии выявлены дополнительные образования в передне-верхнем средостении. Время до диагностики в среднем составило 4 месяца, диапазон – 3–6 месяцев. Средний возраст пациентов составил 23 года, возрастной диапазон – 18–47 лет. Ни в одном случае симптомов возврата опухолевого процесса не было.
С целью уточнения характера выявленного образования в средостении больным выполнялась ПЭТ/КТ с 18F-фтордезоксиглюкозой. У всех пациентов была выявлена гиперплазия тимуса. Характерным признаком, свидетельствующим о гиперплазии ВЖ, является наличие образования в виде V-образной инвертированной формы с диффузным накоплением радиофармпрепарата. SUVmax колебался от 4,0 до 5,5 [12, 15].
Цель исследования: оценить роль ПЭТ/КТ в диагностике гиперплазии тимуса после лекарственной терапии больных лимфомой.
Методы
Нами проведен ретроспективный анализ результатов ПЭТ/КТ с 18F-фтордезоксиглюкозой 103 пациентов с ЛХ и неходжкинскими лимфомами I–III стадий по окончании эффективной ХТ по схемам BEACOPP и R-CHOP.
Возраст больных колебался от 20 до 58 лет, медиана – 36,8 года. Гиперплазия тимуса выявлена у 10 пациентов, что составило 9,7%. Возраст колебался от 26 до 56 лет (медиана – 35,7 года).
Наибольшее число пациентов с гиперплазией ВЖ (8 из 10 – 80%) наблюдалось в возрастной группе до 40 лет; 4 пациента были в возрастном диапазоне от 24 до 29 лет (медиана – 26,8 года); аналогичное количество наблюдалось в возрастной когорте от 34 до 39 лет при медиане возраста 36,3 года. И только одна пациентка была в возрасте 56 лет. Таким образом, полученные нами результаты дают основание говорить о тенденции развития гиперплазии тимуса по окончании ХТ при лимфомах у больных в возрасте до 40 лет.
Среди пациентов с гиперплазией тимуса было 9 женщин и 1 мужчина.
Гиперплазия тимуса наблюдалась у семи пациентов с ЛХ и в трех наблюдениях при неходжкинских лимфомах. По гистологическому строению классическая ЛХ с нодулярным склерозом диагностирована у четырех пациентов, смешанно-клеточный вариант – в трех случаях. Неходжкинские лимфомы были представлены диффузной В-крупноклеточной лимфомой. Исходная распространенность процесса соответствовала II–III стадиям (табл. 1). Пациентам с ЛХ проводили 6 циклов ХТ по схеме BEACOPP, при неходжкинской лимфоме – 6 циклов R-CHOP.
ПЭТ/КТ с 18F-фтордезоксиглюкозой проводилась больным на этапе стадирования каждые 2 цикла на протяжении лечения с оценкой метаболической активности к моменту окончания лечения; затем в процессе динамического наблюдения на протяжении 3–12 месяцев ПЭТ/КТ выполнялась каждые 3–4 месяца. Согласно полученным данным, диагностический диапазон при развитии гиперплазии тимуса колебался от 5 до 12 месяцев, медиана составила 8,4 месяца (табл. 2).
По результатам ПЭТ/КТ, гиперплазия тимуса сопровождается появлением дополнительной ткани в передне-верхнем средостении треугольной формы, обращенной вершиной к грудине. Контуры его ровные, структура, как правило, неоднородная за счет чередования плотности жира и мягких тканей. По нашим данным, неоднородный характер наблюдался в подавляющем большинстве случаев – у 8 пациентов и только у 2 выявлена мягкотканная структура гиперплазированного тимуса.
Метаболическая активность при гиперплазии тимуса имеет диффузный характер фиксации 18F-фтордезоксиглюкозы. Величина стандартизированного коэффициента максимального накопления SUVmax колебалась от 1,98 до 3,28, медиана составила 2,52 (табл. 2).
Ниже приводим ряд клинических наблюдений по диагностике гиперплазии тимуса с помощью ПЭТ/КТ.
Клинический случай 1
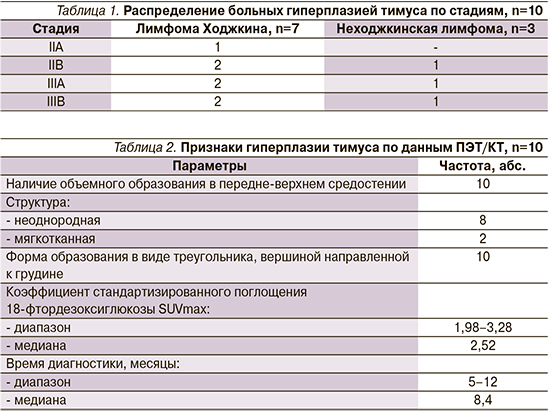
Пациентка В. 36 лет. Диагноз: диффузная В-крупноклеточная лимфома, стадия – IIIВ. Проведено 6 циклов R-CHOP.
Длительность наблюдения – 26 месяцев. По данным динамического наблюдения признаков прогрессирования заболевания нет (рис. 1, 2).
Клинический случай 2
Пациентка Б. 40 лет. Диагноз: классическая ЛХ, нодулярный склероз, стадия – IIIА. Проведено 6 циклов ХТ по схеме BEACOPP.
Длительность наблюдения – 22 месяца. По данным динамического наблюдения, признаков прогрессирования заболевания нет (рис. 3, 4).

Клинический случай 3
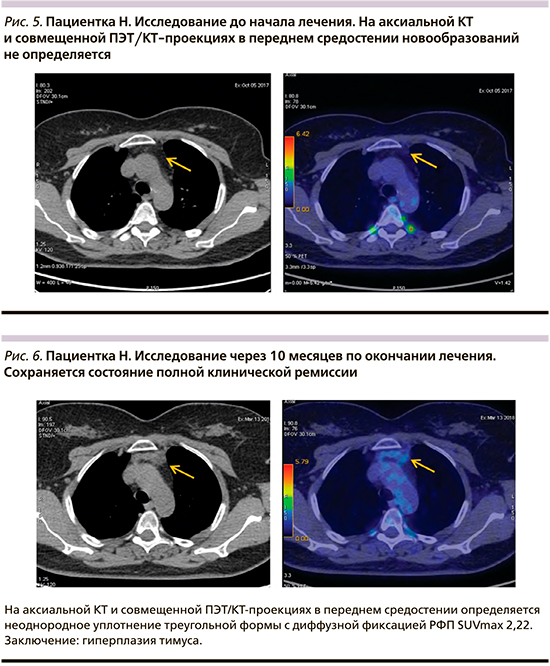
Пациентка Н. 51 года. Диагноз: диффузная В-крупноклеточная лимфома, стадия – IIIА. Проведено 6 циклов R-CHOP.
Длительность наблюдения – 24 месяца. По данным динамического наблюдения, признаков прогрессирования заболевания нет (рис. 5, 6).
Обсуждение
Реактивная гиперплазия тимуса, возникающая во время клинической ремиссии после лекарственного лечения больных лимфомами, сопровождается диффузным его увеличением в виде появления образования в передне-верхнем средостении.
Наличие дополнительного образования в этой области у больных, получивших лекарственное лечение по поводу лимфом, представляет определенную диагностическую дилемму. Прежде всего это может быть расценено как возврат заболевания, но должно быть убедительно подтверждено. Решение этой клинической ситуации нестандартное. Одни авторы предлагают тщательное динамическое наблюдение [16], другие – хирургические вмешательства с целью морфологического уточнения диагноза [14, 13]. Основанием для констатации развития гиперплазии ВЖ служат отсутствие признаков прогрессирования основного заболевания по данным полного обследования при динамическом наблюдении, наличие характерных ПЭТ/КТ-свойств вновь выявленного образования в передневерхнем средостении в сочетании с умеренным повышением поглощения радиофармпрепарата.
В литературе описаны случаи гиперплазии тимуса, в основном развивающиеся при лимфомах, а также при раке молочной железы, саркомах, опухолях яичка [17]. Частота наблюдений ее различна. Чаще всего она наблюдается у лиц молодого возраста. Существует мнение, согласно которому повышенная метаболическая активность тимуса может свидетельствовать о восстановлении его функциональной активности.
C.J. Langer et al. выявили гиперплазию тимуса у 21 больного после ХТ ЛХ. Медиана возраста составила 23 года (возрастной диапазон: 8–40 лет) [18].
T.L. Lin et al. в 2009 г. описали гиперплазию тимуса у 10 пациентов после лечения по поводу ЛХ в возрасте 2–31 года, при этом образование в средостении было выявлено через 6–12 месяцев [19]. Срок развития гиперплазии тимуса, по данным различных авторов, колеблется от 3 до 12 месяцев [13, 14].
Мы наблюдали 10 (9,7%) пациентов с гиперплазией тимуса в возрастном диапазоне 26–56 лет и при медиане 35,7 года. Подавляющее (8 из 10) большинство из них было в возрасте до 40 лет. Среднее время для диагностики составило 8,4 месяца при диапазоне колебаний от 5 до 12 месяцев.
В литературе имеются сообщения о дифференциальной диагностике опухолевых образований в передне-верхнем средостении с применением различных методов медицинской визуализации: рентгеновская компьютерная, магнитно-резонансная томографии и ПЭТ/КТ с 18F-фтордезоксиглюкозой [8, 20].
В 2019 г. Hao-Ran Li et al. опубликовали результаты сравнительного анализа данных литературы с 2009 по 2018 г. о роли КТ и МРТ в диагностике новообразований тимуса. Всего были обследованы 253 больных с использованием КТ и 340 пациентов с применением МРТ. Среди различной патологии у 75 была гиперплазия тимуса.
Авторы показали, что диагностическая чувствительность РКТ и МРТ составила 100%, специфичность – 75 и 80% соответственно. При этом процент правильной постановки диагноза гиперплазии с помощью РКТ составил 84%, превысив остальные нозологические варианты. В то же время с помощью МРТ правильный диагноз гиперплазии тимуса был поставлен в 97,89% случаев, превысив таковую при РКТ. Авторы подчеркивают диагностическое превосходство МРТ над РКТ при постановке диагноза гиперплазии тимуса [21] (см. табл. 3).

В последнее время ПЭТ/КТ с 18-фтордезоксиглюкозой стала востребованным методом метаболической оценки состояния органов и тканей у больных злокачественными новообразованиями, в т.ч. лимфомами. В норме тимус может поглощать 18-фтордезоксиглюкозу у детей и молодых пациентов [22], а увеличение поглощения наблюдается у взрослых после ХТ [10, 15].
По данным ПЭТ/КТ, гиперплазия тимуса характеризуется диффузным и симметричным увеличением его объема с ровными контурами, перемежающейся плотностью жира и мягких тканей. Причем SUVmax в норме имеет низкую интенсивность, составляя примерно от 1,0 до 1,8, при гиперплазии варьируется от 2,0 до 2,8 [23, 24].
C.S. Smith et al. (2007) провели оценку результатов ПЭТ/КТ у 93 детей и молодых людей в возрасте до 20 лет. В 11 (12%) случаях они выявили повышенное накопление ФДГ в ВЖ. Медиана SUVmax составила 2,8, диапазон – 1,1–3,8; у 3 пациентов была ЛХ, в 2 случаях – неходжкинские лимфомы, в 1 – гепатобластома и в 5 – рабдомиосаркома. Одновременно всем пациентам проведена КТ или МРТ. Полученные результаты подтвердили более высокие диагностические возможности ПЭТ/КТ [25].
В нашем исследовании диагноз гиперплазии ВЖ поставлен при использовании ПЭТ/КТ с 18F-фтордезоксиглюкозой после достижения полной клинико-метаболической ремиссии. ВЖ приобретала треугольную форму с вершиной, обращенной к грудине, имела неоднородную структуру у 8 больных и в 2 случаях была мягкотканной. Метаболическая активность носила диффузный характер поглощения 18F-ФДГ, SUVmax колебался от 1,98 до 3,28, медиана – 2,52.
Появление опухоли в средостении у больных лимфомой можно расценивать как остаточную опухоль или признак возврата заболевания. Практическим врачам и онкологам необходимо помнить о столь необычном явлении, как гиперплазия тимуса, возникающая после лекарственного лечения при ряде злокачественных новообразований. Это позволит избегать необоснованного обследования (включая хирургические вмешательства) и проведения противопухолевого лечения.
С учетом диагностической ценности ПЭТ/КТ в трактовке вновь обнаруженных дополнительных образований в передне-верхнем средостении следует подчеркнуть целесообразность обсуждения о внесении изменений в стандартный алгоритм динамического наблюдения пациентов с лимфопролиферативными заболеваниями – использование ПЭТ/КТ в течение первого года после констатации полной клинико-метаболической ремиссии.
На основании литературных данных не представляется возможным высказать мнение о сроках регрессирования выявленной реактивной гиперплазии ВЖ, это требует дальнейшего изучения.
Заключение
Полученные нами данные свидетельствуют о том, что гиперплазия тимуса у больных лимфомами по окончании ХТ встречается в 9,7% случаев и чаще наблюдается в возрасте до 40 лет (медиана – 35,7 года) и при ЛХ. Время ее развития колеблется от 5 до 12 месяцев по достижении полной клиникометаболической ремиссии, составляя в среднем 8,4 месяца. Критерием оценки метаболической активиности при этом служит SUVmax, величина которого в среднем составляет 2,52.
ПЭТ/КТ с 18F-фтордезоксиглюкозой у больных лимфомами служит основной дифференциально-диагностической опцией на этапе развития гиперплазии тимуса и последующего динамического наблюдения.


