Введение
За последние годы значительно возрос интерес к изучению ошибок применения лекарственных средств (ОПЛС) (англ.: medication errors) как причины их нежелательных реакций (НР) и лекарственно-индуцированных заболеваний. Назначение лекарственных препаратов представляет собой сложный процесс, который требует участия различных специалистов в области здравоохранения и происходит в ряде медицинских и фармацевтических учреждений (поликлиника, стационар, аптека и т.д.). Поэтому ОПЛС являются следствием ряда факторов, и эта проблема касается не только врачей, но и медсестер, лаборантов, администраторов медицинских учреждений, фармацевтов и провизоров, производителей лекарственных средств (ЛС), а также самих пациентов, их родственников и/или опекунов. ОПЛС – очень важная и, к сожалению, нередкая причина развития лекарственно-индуцированных заболеваний [1–4].
Определение
Существует несколько определений понятия «ошибка применения ЛС» [1–6]. Согласно одному из них, ОПЛС – это любые непреднамеренные ошибки работника системы здравоохранения, пациента или потребителя при назначении, отпуске, дозировке или введении/приеме лекарственного препарата [2]. Согласно определению Национального координационного совета по отчетности и профилактике ошибок при приеме лекарств NCC MERP (The National Coordinating Council for Medication Error Reporting and Prevention), ОПЛС – это «любое предотвратимое событие, которое может вызвать или привести к неправильному применению ЛС или причинению вреда пациенту, в то время как ЛС находится под контролем медицинского работника, пациента или потребителя» [5].
В Российской Федерации используется определение ОПЛС (медицинской ошибки), представленное в Правилах надлежащей практики Фармаконадзора Евразийского экономического союза: ошибка применения ЛС – это любая непреднамеренная ошибка работника системы здравоохранения, пациента или потребителя в назначении, отпуске, дозировке или введении/приеме ЛС [6]. В этом определении ключевым является слово «непреднамеренная», т.к. в случае, если имеет место преднамеренное действие – намеренное и ненадлежащее использование ЛС, не соответствующее одобренному в инструкции по медицинскому применению, в таком случае – это уже не ОПЛС, а неправильное применение ЛС [6].
Эпидемиология
Частота ошибок при приеме ЛС высока и в развитых, и в развивающихся странах [7, 8], при этом ОПЛС являются причиной развития примерно трети НР ЛС [4, 9]. Частота ОПЛС выше у детей, чем у взрослых, поскольку дозы ЛС рассчитываются для ребенка индивидуально в зависимости от его возраста, массы тела, площади поверхности тела и клинических условий. Кроме того, в педиатрической практике нередки случаи, когда детям назначаются ЛС, которые, согласно инструкции к препарату, не разрешены к применению до определенного возраста [10–12].
Частота ОПЛС в стационаре составляет от 4,8 [13] до 5,3% [14]. ОПЛС являются причиной смерти 1 из 131 амбулаторных пациентов и 1 из 854 госпитализированных больных [15]. По данным FDA (Food and Drug Administration,), примерно 7000 пациентов в США ежегодно умирают вследствие ошибок при использовании ЛС [16]. В Великобритании из-за неправильного приема ЛС в течение 8 лет погибли 29 детей [17]. Наиболее часто ОПЛС ассоциированы с терапией такими препаратами, как инсулин и другие сахароснижающие ЛС, обезболивающие препараты, особенно опиоидные анальгетики, антикоагулянты, другие ЛС для терапии сердечно-сосудистых заболеваний, антибиотики, антигистаминные средства, средства от простуды [18, 19]. Подсчитано, что ОПЛС обходятся системе здравоохранения США в 77 млн долл. ежегодно [20]. ОПЛС увеличивают продолжительность госпитализации в среднем на 4,6 дня [21] и увеличивают стоимость лечения примерно на 2000–2500 долл. на пациента [22].
В Российской Федерации роль медицинских ошибок в развитии НР изучалась В.К. Лепахиным и соавт. [23] при анализе спонтанных сообщений, поступавших в Федеральный центр по изучению побочных эффектов ЛС за 1997–2000 гг. В результате анализа 565 сообщений было установлено, что доля осложнений фармакотерапии вследствие медицинских ошибок составила 27,4%, при этом в 4,2% случаев ОПЛС привели к смерти больного.
Классификация
Всемирная организация здравоохранения (ВОЗ) подразделяет ОПЛС на пять категорий [3, 24, 25]:
1. Нерациональный выбор/назначение ЛС (drug prescribing error): обстоятельства или информация, способные приводить к медицинской ошибке; назначение ЛС при наличии известной гиперчувствительности к нему; использование ЛС пациентами с диагнозом, при котором данный препарат противопоказан согласно указаниям в инструкции к медицинскому применению; ошибка, связанная с межлекарственным взаимодействием, указанным в инструкции к медицинскому применению препарата; ошибка, связанная с взаимодействием ЛС–пища, указанным в инструкции к медицинскому применению препарата; ошибка при вакцинации, в т.ч. при наличии противопоказаний; ошибка, обусловленная схожестью названий/упаковки ЛС; использование ЛС, не разрешенного в данном возрасте согласно указаниям в инструкции к медицинскому применению; выбор неверной дозы ЛС; химическая несовместимость ЛС; фармакологическая или терапевтическая несовместимость ЛС; нерациональное применение ЛС и др.
2. Ошибки отпуска/выдачи (dispensing) ЛС: обстоятельства или информация, способные приводить к ОПЛС; выдача ЛС при наличии известной гиперчувствительности к нему; выдача ЛС, не разрешенного в данном возрасте; ошибка из-за сходства упаковки или маркировки на упаковке с ЛС; ошибка из-за сходства названий ЛС (международных непатентованных или торговых); несоблюдение условий хранения ЛС; отпуск ЛС с истекшим сроком годности; cодержимое не соответствует указанному на упаковке; подозрение на фальсификацию ЛС; выдача ЛС пациенту с диагнозом, при котором данное ЛС противопоказано, что указано в инструкции к медицинскому применению; ошибка, связанная с межлекарственным взаимодействием, указанным в инструкции к медицинскому применению и др.
3. Ошибки производства, хранения и подготовки к использованию (preparation) ЛС: обстоятельства или информация, способные приводить к ОПЛС; ошибка в связи со схожестью упаковки или маркировки на упаковке ЛС; ошибка из-за схожести названий ЛС; применение ЛС с истекшим сроком годности; несоблюдение условий хранения ЛС; использование некачественного ЛС; ошибка в связи с тем, что cодержимое упаковки не соответствует указанному на упаковке; случайное применение неверной дозировки ЛС; неправильная техника в процессе применения ЛС; химическая несовместимость ЛС и др.
4. Ошибки введения (administration) ЛС: обстоятельства или информация, способные приводить к ОПЛС; введение фальсифицированного ЛС; использование неверного медицинского устройства для введения ЛС; введение пациенту ЛС, не разрешенного в данном возрасте согласно инструкции к медицинскому применению ЛС; ошибка из-за схожести упаковки или маркировки на упаковке, ЛС; ошибка из-за схожести названий ЛС; неверный путь введения ЛС; введение неверной дозы ЛС; пропуск дозы; неверный режим приема ЛС; неполный курс вакцинации; введение ЛС с истекшим сроком годности; многократное использование продукта для однократного применения; введение некачественного ЛС; низкая приверженность лечению и др.
5. Ошибки мониторинга (monitoring) медикаментозной терапии: обстоятельства или информация, способные приводить к ОПЛС; неполный курс вакцинации; отсутствие контроля приверженности лечению; непроведение показанного/назначенного диагностического обследования; неверная интерпретация результатов обследования; непринятие мер после получения результатов исследований, отклоняющихся от нормы и др.
По данным Государственного агентства по безопасности пациентов NPSA (National Patient Safety Agency) в Великобритании ОПЛС возникают на всех этапах проведения медикаментозной терапии: 16% при назначении, 18% при распределении и 50% при приеме ЛС. Подобные данные в педиатрии колеблются между 3–37% при назначении ЛС, 5–58% при распределении, 72–75% при приеме медикаментов [17]. По данным Европейского медицинского агентства EMA (European Medicines Agency), в Европе при оказании амбулаторной помощи частота ОПЛС составляет 7,5% на этапе выписки рецептов и 0,08% на этапе отпуска ЛС, в стационарах частота ОПЛС колеблется от 0,3 до 9,1% на этапе выбора и от 1,6 до 2,1% на этапах отпуска и введения ЛС [26].
В систематическом обзоре F.A. Alqenae et al. (2020) изучали причины и частоту медицинских ошибок после выписки пациентов из стационаров и перевода их на ведение специалистами первичного звена здравоохранения, т.е. ошибок, связанных со сменой уровня оказания медицинской помощи [19]. В качестве анализируемых событий выступали: 1) непосредственно медицинские ошибки, 2) неожиданные расхождения в лекарственной терапии (различия в документированных режимах терапии, возникшие между отдельными уровнями и центрами оказания медицинской помощи без обоснования причин этого, могут относиться к ОПЛС или не быть таковыми), 3) НР, 4) побочные эффекты (вред здоровью вследствие медицинской интервенции, связанной с применением препарата), 5) предотвратимые нежелательные побочные эффекты (вред здоровью ввиду ошибочного использования препарата).
В данный систематический обзор были включены исследования, опубликованные в период с января 1990 по март 2019 г., представленные как в электронных реферативных базах данных, так и не входящие в них. Авторы не налагали ограничений относительно языка публикаций и типа изучаемой популяции пациентов. В итоге в систематический обзор вошли 54 исследования, большинство из которых относились к категории среднего (39 работ) и высокого (7 работ) качества научных публикаций (см. таблицу) [19, 27–62].
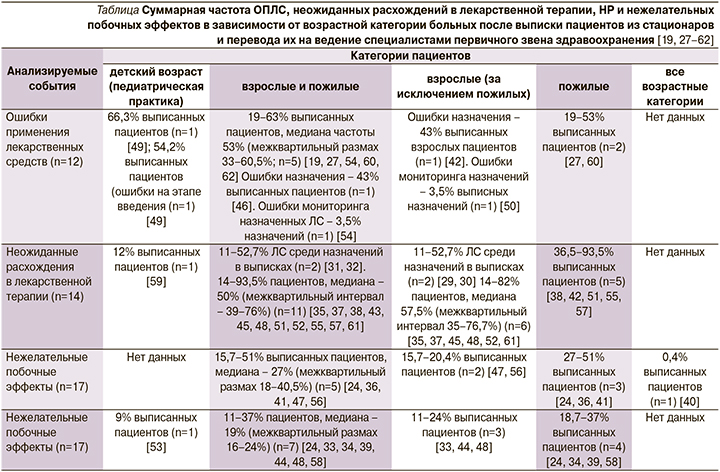
Среди взрослых пациентов медиана частоты ОПЛС и неожиданных расхождений в лекарственной терапии в период после выписки из стационара составила соответственно 53% (межквартильный размах – 33–60,5%; 5 исследований) и 50% (межквартильный размах – 39–76%; 11 исследований). В 5 публикациях имелись данные о частоте НР, медиана которой находилась на уровне 27% (межквартильный размах – 18–40,5%), в 7 работах – сведения о нежелательных побочных эффектах, медиана распространенности последних равнялась 19% (межквартильный размах – 16–24%). В педиатрической практике медицинские ошибки встречались в 66,3% случаев (1 исследование), нежелательные побочные эффекты в 9% (также 1 исследование). Нежелательные побочные эффекты наиболее часто ассоциировались с применением антибиотиков, сахароснижающих ЛС, обезболивающих препаратов и ЛС для терапии сердечно-сосудистых заболеваний [19].
ОПЛС могут оказаться следствием действий не только врачей, но и среднего медицинского персонала (медицинских сестер, лаборантов) и фармацевтов/провизоров. В специальном систематическом обзоре M. Karthikeyan et al. (2015) изучали структуру и причины ОПЛС. В обзор включались все типы исследований, т.е. рандомизированные контролируемые, нерандомизированные контролируемые, проспективные когортные, исследования типа «случай–контроль» и др. [63]. По результатам проведенного обзора литературы авторы отметили, что в доступной литературе в основном присутствуют данные об ОПЛС среди взрослых пациентов. Работы по ошибкам в назначении ЛС имели довольно большие различия по тому, как собраны и описаны данные в большинстве исследований, проведенных в Ближневосточном регионе, анализировали ОПЛС именно на этапе назначения ЛС. Авторы обзора указывают, что частота врачебных ошибок в назначениях ЛС варьируется от 7,1 до 68,2%, из них наиболее часто наблюдаются неправильный учет межлекарственных взаимодействий (68,2%), неполное внесение необходимых данных по назначениям (25%), назначение неверных препаратов (13,0%), ошибки в принципах мониторинга эффективности терапии и состояния пациента (12,6%), выписка недостаточной дозы ЛС (12,6%), внесение некорректных временных интервалов между применением ЛС (12,1%) и передозировка препаратов (7%).
ОПЛС могут также возникать на этапе работы среднего медицинского персонала. Частота таких ошибок, по данным цитируемого систематического обзора [63], колеблется от 19 до 34%. Среди них наиболее распространены нарушения в частоте выдачи/введения препарата (34%), неверное время суток введения (28,6%), неправильная доза (25,3%), введение/выдача/исполнение неполного перечня назначений, что ведет к ситуации, когда пациент получает не все назначенные врачом препараты (24%), неверное применение растворителя для ЛС (22,4%), неверное наименование ЛС (21,2%), неправильный путь введения (19,9%), введение/выдача ЛС одному пациенту, в то время как они предназначены для другого, т.е. ошибки в идентификации пациента (19,7%).
Ошибки могут возникать и на уровне работы фармацевтов. По данным систематического обзора M. Karthikeyan et al. (2015), такая ситуация обнаруживается в 2,29–25% случаев [63]. Сюда относятся ошибки в наименованиях ЛС (25%), передозировки (23%), ошибки маркировки ЛС (23%), в целом указание неправильного режима дозирования (21,83%), ошибки распределения и выдачи препаратов (21%), включая вариант, когда ЛС не назначено врачом, но выдано (20%) и, наоборот, назначено, но не выдано (12,64%), ошибки в приготовлении препарата, что ведет к изменению терапевтического эффекта (10,8%), выдача неправильного количества препарата (6,89%), выдача формы препарата с неверным путем введения (2,29%).
NCC MERP разработал алгоритм для классификации ошибок приема ЛС по 9 категориям в зависимости от степени ущерба, который они могут нанести пациенту [64]:
1. Имели место условия и/или события, которые могли привести к ОПЛС.
2. Ошибка, которая в конечном счете не причинила никакого вреда пациенту.
3. Ошибка, которая совершена пациентом, но не причинила ему никакого вреда.
4. В связи с ошибкой требуется наблюдение за пациентом или какое-либо вмешательство, чтобы убедиться, что ошибка не причинила никакого вреда пациенту.
5. Временная нетрудоспособность пациента. Для устранения последствий ОПЛС требуется какое-либо медицинское вмешательство.
6. Временная нетрудоспособность пациента. Для устранения последствий ОПЛС требуется госпитализация или продление сроков госпитализации пациента.
7. Стойкая утрата нетрудоспособности (инвалидность).
8. Жизнеугрожающее состояние, диктующее необходимость реанимационных мероприятий.
9. Смерть больного.
Факторы, которые ассоциированы с возникновением ОПЛС
NCC MERP объединил все многочисленные факторы, которые влияют на процесс использования ЛС и часто связаны с ошибками их применения в 10 основных факторах (групп факторов): информация, относящаяся к истории болезни пациента, сами ЛС, коммуникация между медицинскими работниками с целью передачи информации о ЛС, номенклатура и упаковка ЛС, хранение, сохранность и стандартизация ЛС, приобретение, использование и мониторинг устройств для приема ЛС, факторы окружающей среды, компетентность и образование медицинского персонала и пациента, процессы управления качеством и рисками [65].
Одним из важных факторов, который недооценивается в качестве фактора риска развития ОПЛС, является тот факт, что пациент принимает не те ЛС, которые записаны в медицинской документации, т.е. может иметь место ошибка, обусловленная неверной или неполной информацией о ЛС, которые реально получает больной. Так, имеются данные, что различия между теми ЛС, которые назначены пациенту, и теми ЛС, которые он реально принимает, могут достигать 87% (!) случаев [3]. Наконец, очень часто в медицинскую документацию не вносят данные о безрецептурных препаратах, которые принимает пациент, в частности в австралийском исследовании было установлено, что запись о назначении безрецептурных ЛС отсутствовали в амбулаторных картах в 74% случаев [66].
В двух последних ситуациях существенно увеличивается риск ошибок, обусловленных неблагоприятными межлекарственными взаимодействиями.
Представляет особый интерес тот факт, что 15–25% и даже более ОПЛС вызваны путаницей из-за схожести названий ЛС, а 33% ошибок – из-за схожести оформления упаковок [67, 68].
Профилактика ОПЛС
С учетом высокой актуальности проблемы ОПЛС не прекращаются попытки разработать стратегии их эффективной профилактики. В 2020 г. опубликован систематический обзор и мета-анализ [69], посвященный сравнению эффективности 12 различных мероприятий (и их комбинаций) по снижению ошибок в назначении, распределении и применении ЛС взрослыми пациентами в условиях терапевтических и хирургических отделений стационаров. Данные мероприятия включали следующие «интервенции»: 1) согласование и выверка фармацевтом применяемых ЛС (мероприятие состоит в идентификации фармацевтом наиболее рационального перечня назначений, включая препарат, его дозу, частоту и способ введения, а также учет потенциальных межлекарственных взаимодействий и обеспечение пациента ЛС из данного списка в госпитальных условиях); 2) использование компьютеризированных систем анализа применяемых ЛС (мероприятие имеет те же задачи и цели, что и предыдущее, однако в отличие от первого варианта для их решения используют специальные компьютерные программы и алгоритмы); 3) компьютеризированная система ввода назначений ЛС, которые сделал лечащий врач, с инструментом поддержки принятия клинического решения или без такового (система позволяет автоматизировать процесс формирования рецептов с наличием унифицированной их формы, требующей обязательного заполнения всех необходимых параметров; инструмент поддержки принятия клинического решение способен предоставлять информацию о наиболее рациональной дозе, пути введения и частоте применения ЛС у конкретного пациента); 4) партнерское сотрудничество с фармацевтами (фармацевт включается в состав полидисциплинарной команды, осуществляющей лечение больного, принимает участие в обходах, мониторирует вводимые ЛС и проверяет листы назначений); 5) обучение врачей, которые назначают пациентам фармакотерапию (в виде on-line-модулей или образовательных мероприятий с фармацевтами); 6) обучение пациентов (с использованием и разъяснением медицинской терминологии, необходимой для правильного понимания пациентом различных аспектов приема ЛС); 7) междисциплинарное сотрудничество (кооперация специалистов здравоохранения различного профиля для выбора наилучшей тактики медикаментозной терапии); 8) привлечение специально подготовленных экспертов в области фармакологии и фармакотерапии; 9) сочетание компьютеризированной системы ввода назначенных врачом ЛС и электронной системы поддержки принятия решения о применении ЛС; 10) автоматизированная система выдачи ЛС (служит для облегчения процесса доведения ЛС до пациента); 11) использование электронного листа назначений (который сочетает инструменты для выписки пациенту ЛС и учета его получения пациентом); 12) применение методов облегчения раздачи ЛС пациентам (заключаются в специальных принципах раскладки ЛС в тележках для их транспортировки по отделению между пациентами, например по кругу, по аналогии с циферблатом часов для облегчения ранжирования времени суток выдачи препарата либо в зависимости от их названия).
В анализ не включались исследования, оценивавшие результаты таких мероприятий в амбулаторных условиях, в отделениях неотложной и интенсивной терапии, а также исследования, в которых изучали вопросы медицинской помощи в периоперационном периоде, у пациентов неврологического и онкологического профилей [69].
В данные систематический обзор и мета-анализ [69] вошли 34 исследования, найденные в результате поиска по 6 базам данных научных публикаций (MEDLINE, CINAHL, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials) в период от момента их создания и до февраля 2019 г., объем выборок пациентов в исследованиях варьировался от 33 до 1852 человек. В 14 исследованиях продемонстрировано снижение частоты ошибок при назначении и выписке ЛС, в 4 работах – уменьшение числа ошибок в применении и введении ЛС. Ни в одной из 34 публикаций не было достигнуто снижения частоты ошибок в распределении и выдаче препаратов.
В 6 исследованиях изучался эффект согласования и выверки фармацевтом применяемых ЛС на частоту ошибок в назначении ЛС. В 2 из 6 исследований такое мероприятие позволило снизить частоту ошибок данного рода и их последствий. Так, A. Al-Hashar et al. [50] обнаружили снижение числа потенциально предотвратимых НР с 16 до 9,1% (р=0,008). В другой работе [70] относительное число пациентов, у которых обнаружены ошибки в выписке ЛС, после внедрения данной тактики контроля врачебных назначений снизилось с 35,1 до 16,7%. В пилотном рандомизированном контролируемом исследовании [71] показано снижение числа непреднамеренных ошибок с 2,71 на пациента в контрольной группе до 0,02 ошибки на больного в основной группе (согласование и выверка фармацевтом применяемых ЛС).
В двух исследованиях изучалось влияние использования компьютеризированных систем анализа применяемых ЛС на частоту ОПЛС, сохранявшихся на момент выписки пациента из стационара, и только в одном из них было выявлено статистически значимое уменьшение числа таких ошибок. В исследовании G.M. Allison et al. [72] проводился ретроспективный анализ наличия ошибочного назначения внутривенно применяемых антибиотиков пациентам в рекомендациях при выписке до и после использования компьютерной программы анализа и согласования применения антибактериальных препаратов. В результате авторы обнаружили, что такая интервенция снизила число пациентов как минимум с одной ошибкой на момент выписки с 23 до 11% (значение р в публикации не приводятся). В другом исследовании [73] обнаружено статистически значимое (р<0,001) уменьшение числа ОПЛС на момент выписки с 645 до 359 и несоответствий между назначением и фактическим характером использования ЛС с 3490 до 2823. Вместе с тем в данной работе [73] не было обнаружено статистически значимого изменения в числе ошибок с потенциальными серьезными/жизнеугрожающими последствиями на фоне использования компьютеризированных систем анализа применяемых ЛС: до интервенции их число составляло 1,4%, после – 3,1% (р=0,10).
В одном исследовании [74] анализировался эффект согласования и выверки назначаемых ЛС квалифицированным куратором (англ.: trained mentor). В данной работе принимали участие пять центров: в трех из них проводилась указанная интервенция, остальные два служили контрольными центрами. Конечной точкой стало число нарушений в структуре назначений на момент поступления и выписки, которые могли принести потенциальный вред пациенту. Финальный анализ по интересующей интервенции проводился в двух центрах – в обоих на ее фоне наблюдалось снижение числа ошибок в пересчете на одного пациента с 1,00 до 0,88 и с 0,30 до 0,18 (значение p авторы не приводят).
Эффект компьютеризированной системы ввода назначений врачей на ОПЛС изучался в пяти работах, и во всех из них при применении такого подхода обнаружено статистически значимое снижение ОПЛС.
F. Hernandez et al. [75] провели наблюдательное исследование с оценкой числа таких ошибок до и после использования компьютеризированной системы ввода назначений ЛС лечащими врачами без модуля поддержки принятия клинического решения. Авторы пришли к выводу, согласно которому такой подход обеспечивал статистически значимое (p<0,0001) снижение числа ошибок в назначении ЛС с 30,1 до 2,4%, а число ошибок в распределении ЛС между больными не изменилось. В другой работе [76] изучалось влияние компьютеризированной системы ввода назначений врачей с наличием поддержки принятия клинического решения среди пациентов с хронической болезнью почек, которые поступили в стационар в связи с острым коронарным синдромом. В группе интервенции не было выявлено использования ЛС вопреки наличию противопоказаний к ним (ни одного случая среди 33 пациентов), в то время как в контрольной группе такая ситуация наблюдалась у 8 больных из 47 (р=0,01 между группами). Еще в одном исследовании [77] использование подобной компьютеризированной системы привело к статистически значимому снижению числа ошибок в назначениях ЛС – с 50,2 до 28,2%. R. Shawahna et al. [78] при сравнении частоты ошибок в назначении ЛС пришли к следующему выводу: рассматриваемая интервенция позволяет снижать их число с 22,6 до 8,2% (р=0,01). Наконец, еще в одном исследовании [79] с дизайном по типу анализа прерванных временных рядов (ситуация, когда интересующий параметр рассчитывается до и после определенной интервенции в одном и том же ряду субъектов) использование компьютерной системы ввода назначений врачей способствовало снижению числа ошибок в назначении ЛС с 63,3 до 18,8% (значения р авторы не приводят).
Эффективность стратегии партнерского сотрудничества с фармацевтами изучалась в трех исследованиях и во всех выявлено статистически значимое снижение числа ошибок в назначении ЛС на фоне этой интервенции. Так, C. García-Molina Sáez et al. [80] анализировали эффект включения в лечащую команду фармацевта, который вносил ЛС, назначенные пациенту перед госпитализацией, в электронную версию истории болезни. В последующем компьютерный алгоритм, интегрированный в электронную историю болезни, анализировал правильность назначений ЛС лечащими врачами. Такой подход позволил снизить число ошибок в одобренных схемах терапии с 47,7 до 17,3% (р<0,001). В другой работе [81] фармацевты привлекались к врачебным обходам пациентов. На фоне данной интервенции число ОПЛС (27,5%) оказалось статистически значимо ниже (p<0,001) по сравнению с тем же показателем до применения данного подхода (53,0%). Еще в одном исследовании [82] мониторирование фармацевтом назначений ЛС лечащими врачами привело к уменьшению ошибок в назначении ЛС среди серопозитивных пациентов с вирусом иммунодефицита человека с 85 случаев на 330 пациентов до 24 на 330 больных (р<0,001).
Эффективность стратегии обучения врача, формирующего назначения ЛС по специальным обучающим программам в плане снижения частоты ОПЛС, изучалась в одном исследовании с применением кластерного рандомизированного анализа [83]. Согласно его дизайну, было сформировано три группы молодых врачей: 1) контрольная, 2) группа с оценкой знаний врачей и последующим адресно-ориентированным обучением при участии фармацевта и 3) группа с применением электронных методик обучения. В группе с привлечением в качестве преподавателя фармацевта еженедельно на протяжении 4 недель проводились три 10-минутных сессии с детальным обсуждением недавних ошибок в назначении ЛС. Врачи 3-й группы (дистанционные методики обучения) проходили образовательный on-line-курс по практике рационального и безопасного назначения ЛС. В результате установлено, что в контрольной группе и в группе on-line-обучения произошло статистически значимое увеличение (!) числа ошибок в назначении ЛС – соответственно с 49,0 до 58,8% (р<0,001) и с 58,2 до 63,3% (р=0,025). В отличие от этого в группе обучения с участием фармацевтов число ошибок, наоборот, снизилось с 57,8 до 37,0% (р<0,001). В рассматриваемой работе также регистрировались клинически значимые ошибки в назначении ЛС (включая потенциально ведущие к летальным исходам, серьезные и существенные). Их число на фоне обучения с участием фармацевта статистически значимо не менялось (р=0,068).
Эффект обучения пациентов на частоту ОПЛС изучался также в одной работе – рандомизированном контролируемом пилотном исследовании [84]. В его рамках пациенты получали копию своего листа назначений с наличием глоссария, разъясняющего общепризнанные медицинские термины. Относительное число пациентов с наличием потенциальных и явных НР статистически значимо не различалось между группой вмешательства и контрольной группой (p=0,30 и 0,22).
В 4 исследованиях оценивалось влияние привлечения специально подготовленных экспертов в области фармакологии и фармакотерапии на частоту ОПЛС, и в одном из них такой подход обеспечил статистически значимое снижение процента ошибок. Так, W. Baqir et al. [85] изучали эффект привлечения фармацевтов со специализированным образованием для поддержки решений врачей в условиях стационара. Данная интервенция обеспечивала статистически значимое (р<0,0001) снижение числа ОПЛС до 2 случаев на 168 пациентов по сравнению с контрольной группой (68 случаев на 369 пациентов). Вместе с тем в другом исследовании [86] сходный подход с привлечением обученных медсестер не привел к статистически значимым изменениям в числе ОПЛС (р=0,84): их частота в группе интервенции и в контрольной группе составила 15,7 и 14,9% соответственно. В другой работе [87] снижения числа ОПЛС пытались добиться посредством обучения медсестер детальной проверке рецептов, особому вниманию к правильному применению у пациентов назначенных ЛС, проверке маркировки ЛС, регистрации введения/выдачи препарата пациенту и минимизации случаев прерывания терапии. В результате такого подхода число ОПЛС снизилось до 0 на 100 назначен,ий, однако, насколько это значимо, утверждать сложно, поскольку исходная частота ОПЛС была весьма низкой (2 случая на 100 назначений). P.J. Schneider et al. [88] выполнили рандомизированное контролируемое исследование по влиянию обучения медсестер на частоту ОПЛС, к сожалению, анализируемый подход к снижению частоты ОПЛС не привел к статистически значимым результатам (отношение шансов [ОШ]=1,92, 95% доверительный интервал [ДИ]: 0,81–4,58; р=0,14).
В 2 исследованиях изучались различные подходы к выдаче ЛС пациентам. В одном из них [89] сравнивался традиционный метод выдачи препаратов в рамках госпитализации персоналом стационара и стратегия, когда пациент принимал собственные препараты, привезенные из дома (назначенные амбулаторно). Авторы не обнаружили различий в частоте ОПЛС на фоне традиционного подхода (4,3%) и варианта приема больным собственных препаратов (4,2%; р=0,99). В другом исследовании [90] в условиях ортопедического отделения листы назначений заполнялись в алфавитном порядке и такой подход сравнивался с рутинным заполнением, когда листы заполнялись в соответствии с периодичностью приема в течение суток. В последней работе также не было обнаружено снижения частоты ОПЛС на фоне нового подхода к выдаче препаратов (19,4% при рутинном подходе к выдаче ЛС и 23,0% после перехода на заполнение листов назначения в алфавитном порядке, ОШ=1,24, 95% ДИ: 0,95–1,62).
В одной работе [91] анализировался эффект комбинированного вмешательства – применения автоматизированной системы выдачи ЛС в сочетании с использованием электронного листа назначений/без такового. Было показано, что оба подхода обеспечивают снижение числа ОПЛС: исходная частота ОПЛС составляла 10,6%, при использовании автоматизированной системы выдачи ЛС без электронного листа она снизилась до 5,8% (р=0,02), а при использовании еще и электронного листа назначений частота ОПЛС уменьшилась еще больше – до 4,1% (р=0,001).
В опубликованных работах также оценивалось влияние других различных комбинированных подходов, из них наиболее частым являлось сочетание обучения лечащего врача, партнерское сотрудничество с фармацевтом и использование компьютеризированной системы ввода назначений лечащих врачей. В таких исследованиях получены неоднозначные результаты. T.C. Grimes et al. [92] изучали эффект согласования и выверки фармацевтом применяемых ЛС в сочетании с партнерским сотрудничеством с ним лечащего врача при терапии острых состояний. Авторы выявили, что такие мероприятия позволяют статистически значимо снизить частоту ошибок в назначении ЛС (13,9% в группе интервенций, 65,3% в контрольной группе; p<0,0001).
В другой работе [93] получены сходные результаты: комбинация обучения лечащего врача в сочетании с согласованием и выверкой фармацевтом применяемых ЛС эффективна в снижения числа ошибок в назначении ЛС. Вместе с тем L.M. Daneils et al. [94] не обнаружили статистически значимого влияния комбинации обучения лечащего врача с использованием компьютеризированных систем ввода рецептов на частоту ошибок в назначении ЛС. В упоминавшейся выше работе [95] сочетание обучения врача, формирующего назначения, и партнерского сотрудничества с фармацевтом не привело к статистически значимому снижению числа ОПЛС в условиях отделения неотложной хирургической помощи – число ОПЛС до интервенции составляло 12,0 на 100 тыс. пациенто-часов, после – 10,9 на 100 тыс. пациенто-часов (относительный риск – ОР=0,92, 95% ДИ: 0,40–2,08; р=0,835). Также анализировалось [96] влияние использования компьютеризированной системы анализа применяемых ЛС, интегрированной в систему ввода рецептов врачей, в отделении общехирургического профиля. Такие мероприятия все же способствовали статистически значимому снижению числа непреднамеренных ошибок с 10,6 до 6,6% (р=0,002).
Кроме того, имеются данные [97], согласно которым сочетание компьютеризированной системы ввода рецептов врачей, обучения врачей и междисциплинарного сотрудничества способствует снижению ОПЛС у пациентов с вирусом иммунодефицита человека, поступивших с острыми состояниями в отделения терапевтического и хирургического профиля (с 50 до 34%; p<0,001). Влияние комбинированных интервенций на ошибки в распределении ЛС изучалось лишь в одной работе [94]. В ней сочетание применения компьютеризированной системы ввода рецептов врачей и обучения лечащих врачей привело к снижению частоты ошибок в распределении ЛС с 33 до 24%, однако полученные результаты не достигли статистической значимости (ОР=0,72, 95% ДИ: 0,29–1,76).
Таким образом, существуют как отдельные стратегии, так и их сочетания, которые в соответствующих исследованиях продемонстрировали свою эффективность в снижении числа ОПЛС, поэтому должно быть рассмотрено их широкое внедрение в клиническую практику. Важным и эффективным принципом в контексте выбора наиболее рациональных и хорошо организованных стратегий медикаментозного лечения в плане уменьшения ОПЛС является работа специалистов в виде полидисциплинарной команды с тесным взаимодействием между врачами (в т.ч. с привлечением клинических фармакологов), средним медицинским персоналом и фармацевтами.
Заключение
Из представленных данных можно сделать вывод: ОПЛС представляют собой одну из ведущих причин осложнений, связанных с медикаментозной терапией, в т.ч. являясь значимым фактором риска развития лекарственно-индуцированных заболеваний.
В настоящее время распространенность ОПЛС остается довольно высокой, а сами они могут возникать на различных этапах организации лекарственной терапии, начиная от формирования назначения лечащим врачом и заканчивая непосредственно введением препарата в организм пациента. В силу высокой значимости ОПЛС в возникновении ятрогений ведутся поиск, разработка и анализ эффективности мероприятий по снижению частоты таких ошибок, и, согласно доступной литературе, определенные успехи в этом вопросе прослеживаются. Но, несмотря на это, невозможно не отметить, что на сегодняшний день требуется дальнейшая активная научно-исследовательская и практическая работа по максимально возможной минимизации числа ОПЛС ввиду их принципиальной роли в аспектах безопасности лекарственной терапии.


