Введение
По состоянию на июль 2022 г. более 567 млн человек были инфицированы SARS-CoV-2 во всем мире и 6,3 млн умерли [12]. Частота респираторных и функциональных нарушений после COVID-19 все еще обсуждается, но в нескольких исследованиях обнаружено уменьшение объема легких, снижение диффузионной способности легких и снижение толерантности к физической нагрузке после выписки из больницы [13, 16]. Одним из осложнений пневмонии COVID-19 и острый респираторный дистресс-синдром (ОРДС) является фиброз легких [5]. Хотя в настоящее время нет клинических данных о частоте и механизме легочного фиброза после COVID-19; по оценкам, он затрагивает около трети пациентов, госпитализированных с SARS-COV-2 [4, 5, 7]. Это указывает на то, что совокупная распространенность среди госпитализированных и не госпитализированных пациентов может быть еще больше.
Поскольку примерно у 30% выживших после тяжелого острого респираторного синдрома 2003 г. и ближневосточного респираторного синдрома наблюдались стойкие рентгенологические и физиологические нарушения, соответствующие фиброзной болезни легких, следует ожидать, что последствия COVID-19 могут включать формирование у большой когорты лиц легочного фиброза и стойких потенциально прогрессирующих физиологических нарушений [6]. Развитие этого осложнения сопряжено с большими экономическими потерями в связи с временной утратой трудоспособности пациентов, перенесших SARS-CoV-2-инфекцию, сокращением потенциального дохода пациента и родственников, а также другими медицинскими расходами, например, на реабилитацию.
Еще одним заболеванием, сопряженным с развитием фиброза легких, является идиопатический легочный фиброз, распространенность которого в Российской Федерации составляет около 8–12 случаев на 100 тыс. населения, а заболеваемость – 4–7 случаев на 100 тыс. населения [3, 17].
В основе патогенеза легочного фиброза (как идиопатического, так и SARS-Cov-2-ассоциированного) лежат микроповреждения альвеолярного эпителия с нарушением механизмов его регенерации. Это приводит к патологической реэпителизации, пролиферации фибробластов и синтезу избыточного количества экстрацеллюлярного матрикса. В результате нормальная легочная паренхима постепенно замещается фиброзной тканью [1, 2].
Потенциальная роль антифибротической терапии предлагается на основе в основном неофициальных данных и предполагаемого сходства механизма фиброза легких после COVID-19 с идиопатическим легочным фиброзом [6]. К ведущим препаратам, имеющимся в терапевтической практике, рассматриваемым как потенциально эффективные, относятся пирфенидон и нинтеданиб. Пирфенидон изучается в настоящее время в качестве антифибротического средства после COVID-19 с РКИ [8]. Однако пирфенидон имеет только одного отечественного производителя (оригинальный препарат имеет зарубежное производство), а нинтеданиб производятся только за границей, оба имеют достаточно высокую стоимость, а их применение может быть ассоциировано с потенциальной гепатотоксичностью, что особенно нежелательно, поскольку дисфункция печени часто встречается у пациентов, инфицированных SARS-CoV-2 [9, 10]. В качестве других кандидатов рассматриваются тетрандин [18], фучжэнхуаю (капсулы) в комбинации с ацетилцистеином [19], анлуохуаксан [20], амниотическая жидкость человека [21], мезенхимальные стволовые клетки [22], гипербарический кислород [23] и др. В этих условиях поиск новых высокоэффективных и безопасных соединений, способных предотвращать или разрешать развитие фиброза легких, остается актуальной задачей. Исследование, описанное в статье Г.С. Аникина и др. [34], показало, что применение Ингасалина® форте 7% через небулайзер в составе комплексной терапии в 3 раза быстрее по сравнению со стандартной терапией нормализует показатели сатурации уже на 5-й день лечения и значительно уменьшает одышку у пациентов с COVID-19. Цель исследования: изучение эффективности медицинского изделия на основе гипертонического раствора натрия хлорида (7%) и натрия гиалуроната (0,1%) [Ингасалин® форте] на блеомициновой модели фиброза легких.
Методы
Исследование проведено на 30 аутбредных крысах-самцах массой 180–200 г, полученных из ФГУП ПЛЖ «Рапполово» (Ленинградская обл.). Содержание животных и все исследования проводили согласно требованиям решения Совета Евразийского Экономического Союза в сфере обращения лекарственных средств, Приказом Минздрава РФ № 199н «Об утверждении правил надлежащей лабораторной практики» от 01.04.2016, методическим указаниям по содержанию и использованию лабораторных животных (Guide for the care and use of laboratory animals. National Academy press; Washington, D.C., 1996) и Директивой 2010/63/EU Европейского парламента и Совета Европейского Союза от 22.09.2010 года по охране животных, используемых в научных целях и с одобрения биоэтической комиссии ФГБОУ ВО СПХФУ Минздрава России (протокол биоэтической комиссии Rats-01-FIB-22).
Было сформировано 3 группы животных по 10 особей в каждой. Экспериментальную модель фиброза легких создавали однократным интратрахеальным введением животным блеомицина (NIPPON KAYAKU, Co. Ltd., Japan) в дозе 5 мг/кг [11]. В первую группу вошли интактные животные. Вторая группа – животные с экспериментальным фиброзом легких (патология без лечения). Животные третьей группы на фоне патологии получали ингаляции со 2-х суток после введения блеомицина ежедневно (2 раза в день) в течение 28 дней медицинского изделия Ингасалин® форте на основе гипертонического раствора натрия хлорида (7%) и натрия гиалуроната (0,1%) в объеме 5 мл/камеру (42,7 л) с помощью небулайзера Delphinus F1000 (Flaem Nuova, Италия).
Расчет доз препаратов для введения животным проводили при помощи коэффициентов пересчета доз для крысы и человека в зависимости от массы тела по методике E.J. Freireich (1966) [24] на основании данных инструкции по медицинскому применению.
На 30-й день животных выводили из эксперимента и проводили некропсию. Легкие и сердце извлекали, помещали в 10%-ный забуференный формалин и фиксировали в течение 24 ч. Образцы тканей подвергали стандартной гистологической проводке в гистопроцессоре Thermo Scientific Excelsior AS (Thermo Shandon Limited, Англия), после чего заливали в парафин, на ротационном микротоме готовили срезы толщиной 3,0–3,5 мкм, помещали их на предметные стекла и окрашивали гематоксилином и эозином. Аналитический этап работы проводили с использованием проходящего света и микроскопа Axio Scope A1 (Carl Zeiss, Германия).
Оценивали наличие интерстициальной пневмонии, десквамативной пневмонии и фиброза.
Статистическую обработку полученных данных проводили с использованием программы Prism 9.0.0 (GraphPad Software, США), значимость различий между распределениями частот встречаемости шкалированных гистологических оценок определяли с помощью расширенного точного теста Фишера с поправкой Холма–Бонферрони для множественных сравнений.
Результаты и обсуждение
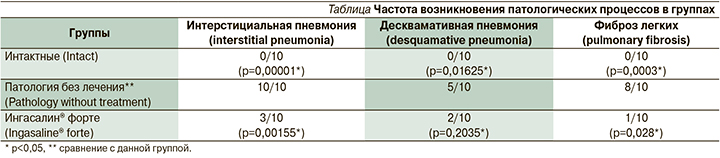
В проведенном исследовании интратрахеальное введение блеомицина привело к развитию интерстициальной пневмонии у всех животных в группе патологии без лечения, у 50% животных развилась десквамативная форма пневмонии, а пневмофиброз был выявлен у 8 из 10 животных.
Использование медицинского изделия [Инагасалин® форте 7%] у животных третьей группы на фоне патологии привело к выраженному терапевтическому эффекту: произошло снижение частоты развития интерстициальной пневмонии на 70%, десквамативной на 60% и фиброза на 87,5%.
Гистологическим исследованием установлено отсутствие патологических изменений легких у животных из группы интакта (рис. 1А), развитие выраженного диффузного пневмофиброза в исходе интерстициальной пневмонии (рис. 1Б) у животных из группы «Патология без лечения». Обычная интерстициальная пневмония характеризовалась неравномерным утолщением межальвеолярных перегородок за счет отека, очаговой или диффузно-очаговой инфильтрации их лимфоцитами, формированием разрастаний грануляционной ткани разной степени зрелости и неравномерно выраженным фиброзом межальвеолярных перегородок со снижением воздушности легочной ткани (рис. 1В). Такие изменения носили фокальный, мозаичный характер и имели разную степень выраженности. Для десквамативной интерстициальной пневмонии была характерна распространенная десквамация клеток альвеолярного эпителия в просветы альвеол наряду с патологическими изменениями межальвеолярных перегородок в виде отека, инфильтрацией лимфоцитами и фиброза разной степени выраженности со значительным снижением воздушности легочной ткани (рис. 1Г).
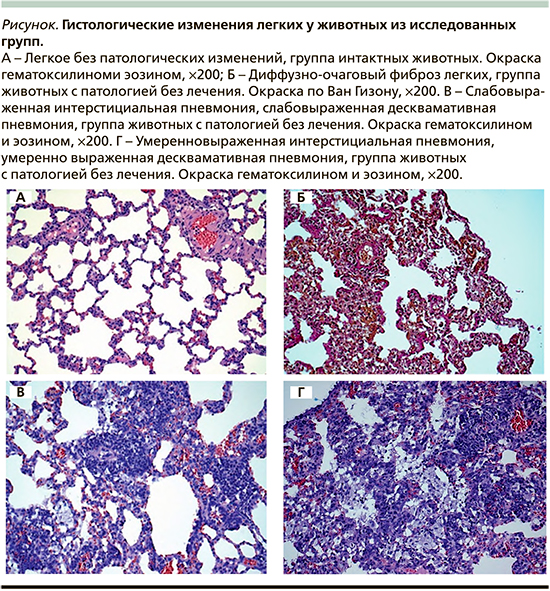
По результатам гистологического анализа установлена частота возникновения патологических процессов в группах; данные представлены в таблице.
Вспомогательные средства для очищения дыхательных путей, такие как гиперосмолярные средства, изменяют вязкость мокроты и/или усиливают мукоцилиарный клиренс. Установлено, что 7%-ный гипертонический раствор натрия хлорида вызывает улучшение реологических свойств бронхиального секрета и облегчает его эвакуацию за счет нескольких механизмов. Благодаря наличию плотной гидратной оболочки у ионов натрия происходит активная регидратация бронхиального секрета вследствие усиления осмоса воды под действием ионов, содержащихся в растворе [25]. Это также способствует снижению вязкости и адгезивности бронхиального секрета.
Эффективность гипертонических растворов установлена в нескольких исследованиях, в т. ч. у больных муковисцидозом, у которых ингаляции 7%-ного раствора натрия хлорида вызывали улучшение качества жизни и снижение частоты обострений [26].
В клиническом исследовании у пациентов с бронхоэктазами, не связанными с муковисцидозом, применение 7%-ного раствора натрия хлорида в дополнение к физиотерапии по сравнению с физиологическим раствором привело к достоверному увеличению массы отделяемой мокроты, уменьшению ее вязкости и облегчению отделения мокроты. Эти благоприятные изменения ассоциировались с более выраженным увеличением объема форсированного выдоха за 1 секунду (ОФВ1) у пациентов, получавших гипертонический раствор [27].
Те же авторы опубликовали результаты длительного рандомизированного перекрестного исследования, в котором сравнивали эффективность введения изотонического физиологического и гипертонического (7%) растворов для больных бронхоэктазами [28]. Применение гипертонического раствора по сравнению с изотоническим физиологическим раствором привело к увеличению ОФВ1 и форсированной жизненной емкости легких, улучшению качества жизни, а также к снижению частоты применения антибиотиков и обращений за неотложной помощью. Кроме того, при применении гипертонического раствора отмечено уменьшение вязкости мокроты и улучшение ее отделения.
Для улучшения переносимости гипертонического раствора предложено использовать гиалуроновую кислоту (ГК), которая усиливает гидратирующий эффект препарата, предупреждает бронхоконстрикцию, стимулирует движения ресничек и уменьшает неприятный вкус гипертонического раствора [29].
ГК является ключевым компонентом внеклеточного матрикса легких. Уникальным свойством ГК являются ее влагоудерживающие свойства, поэтому ГК играет важную роль в регуляции баланса жидкости в интерстиции легких. ГК широко используется при лечении различной патологии, но в последние годы она также была предложена для лечения некоторых заболеваний легких, включая заболевания дыхательных путей, благодаря своим противовоспалительным и водосвязывающим свойствам. Аэрозоль ГК предотвращает бронхоконстрикцию у астматиков и улучшает некоторые функциональные параметры у пациентов с хронической обструктивной болезнью легких (ХОБЛ).
Благодаря защите ГК от бронхоконстрикции и ее гидратационным свойствам вдыхаемая ГК увеличивает объем жидкости на поверхности дыхательных путей, что приводит к гидратации бронхиального секрета, усилению его транспорта и уменьшению закупорки дыхательных путей слизью. Кроме того, в клинических исследованиях показано, что лечение ингаляционной ГК улучшает переносимость ингаляционно вводимого гипертонического физиологического раствора (даже при концентрации 6 или 7%), что, как было продемонстрировано, служит эффективным средством для контроля бронхиальной секреции у пациентов с муковисцидозом и бронхоэктазами [30].
Сходные результаты получены в других исследованиях, в которых сравнивали эффективность и переносимость комбинации 7%-ного гипертонического раствора и ГК [31, 32].
Данная комбинация имеет ясные [33] перспективы при острых воспалительных процессах в легких, когда фиброз только инициируется.
Заключение
Проведенным исследованием установлено, что использование ингаляционного медицинского изделия Ингасалин® форте на модели блеомицинового фиброза легких у крыс позволяет уменьшать выраженность альтерационных и фибротических изменений в органе, вызываемых токсикантом.
По сравнению с группой патологии без лечения в группе терапии медицинским изделием Ингасалин® форте частота развития интерстициальной пневмонии была в 3,3 раза меньше, частота развития десквамативной пневмонии – в 2,5, а частота развития фиброза легких в 8,0 раз меньше.
Таким образом, ежедневное (2 раза в день в течение 28 дней) ингаляционное введение медицинского изделия Ингасалин® форте 7% оказало положительное влияние, способствовавшее сдерживанию развития патологического процесса.
Вклад авторов. Е.Д. Семивеличенко – проведение эксперимента, обобщение данных, написание статьи. Д.Ю. Ивкин – обзор литературных источников, обобщение данных, написание статьи. С.В. Оковитый – обобщение данных, написание статьи. В.Е. Карев – гистологический анализ данных.
Финансирование. Результаты работы получены с использованием оборудования ЦКП «Аналитический центр ФГБОУ ВО СПХФУ Минздрава России» в рамках соглашения № 07515-2021-685 от 26 июля 2021 г. при финансовой поддержке Минобрнауки России, статья подготовлена при финансовой поддержке Solopharm.



