Введение
Препараты класса ингибиторов натрий-глюкозного ко-транспортера 2-го типа (иНГЛТ-2) в течение длительного времени применялись как сахароснижающие. Родоначальником данного класса препаратов стал флоризин, который был выделен из коры яблони в 1835 г. и вначале применялся для лечения лихорадки, малярии и других инфекционных заболеваний. Глюкозурический эффект препарата был выявлен много лет спустя [1].
В России для лечения пациентов с СД2 в 2014 г. были зарегистрированы дапаглифлозин и эмпаглифлозин, в 2015 г. – канаглифлозин, в 2019 г. – ипраглифлозин и эртуглифлозин.
В основе сахароснижающего эффекта данного класса препаратов лежит преимущественное селективное обратимое ингибирование натрий-глюкозного ко-транспортера 2-го типа (НГЛТ-2), приводящее к снижению реабсорбции глюкозы и натрия из клубочкового фильтрата в почечных канальцах, выведению глюкозы и натрия с мочой и осмотическому диурезу [2, 3].
Как известно, в норме вся отфильтрованная в почечных клубочках глюкоза подвергается реабсорбции в почечных канальцах, поэтому глюкоза в моче не присутствует [5]. НГЛТ-2 расположен в начальном сегменте S1 проксимальных почечных канальцев и реабсорбирует порядка 80–90% отфильтрованной глюкозы. НГЛТ-1, расположенный в сегменте S2/S3 проксимального канальца, реабсорбирует оставшиеся 10–20% [6]. В α-клетках поджелудочной железы и в мозжечке был обнаружен НГЛТ-2, тогда как НГЛТ-1 более широко распространен в почках, кишечнике, сердце, легких и скелетных мышцах. При концентрации глюкозы в плазме выше порога глюкозурии 10 ммоль/л фильтрованная глюкоза начинает выводится с мочой. У пациентов с СД наблюдается повышение порога глюкозурии и возрастание реабсорбции глюкозы в почках. Последняя связана с усилением экспрессии НГЛТ-2 с целью предотвращения потери глюкозы как энергетического субстрата и вносит свой вклад в многофункциональный патогенез гипергликемии при СД [6, 7]. Ингибирование НГЛТ-2 препаратами класса иНГЛТ-2 имеет инсулин-независимый характер и не связано с инсулин-секретирующей способностью β-клеток поджелудочной железы, что позволяет продолжать их применять в т.ч. и пациентам с истощенным инсулярным аппаратом после потери эффективности стимуляторов секреции инсулина – ингибиторов дипептидилпетидазы 4-го типа (иДПП4), агонистов рецепторов глюкагоноподобного пептида-1 (арПП-1), препаратов сульфонилмочевины и меглитинидов [5, 8].
Результаты клинических исследований иНГЛТ-2
Глюкозоснижающий эффект иНГЛТ-2 зависит от концентрации глюкозы в плазме, а также от скорости клубочковой фильтрации (СКФ). Поэтому эффективность индуцированной препаратом глюкозурии будет выше у лиц с высокой гипергликемией при относительно сохранной функции почек. Сахароснижающие эффекты иНГЛТ-2 уменьшаются у пациентов с рСКФ <60 мл/мин/1,73 м2 и почти отсутствуют при рСКФ <30 мл/мин/1,73 м2 [4].
В связи с этим целесообразно добавление препаратов класса иНГЛТ-2 в схему сахароснижающей терапии СД2 на ранних стадиях заболевания, когда функция почек у пациентов еще сохранена и рСКФ имеет более высокие показатели [10].
По данным систематического обзора и мета-анализа, в который были включены 45 исследований сравнения иНГЛТ-2 с плацебо (n=11 232) и 13 исследований сравнения иНГЛТ-2 с активными препаратами (n=5175), показано, что иНГЛТ-2 снижают уровень HbA1c у пациентов с СД2 [11].
Дапаглифлозин, первый представитель класса иНГЛТ-2, который был зарегистрирован на территории РФ, подробно изучен у пациентов с СД2. Программа исследований дапаглифлозина позволила получить убедительные данные об эффективности и безопасности препарата для улучшения гликемического контроля как в качестве монотерапии, так и для комбинированного применения с метформином, производными сульфонилмочевины, тиазолидиндионами, иДПП-4, арГПП-1 (эксенатидом пролонгированного действия) и препаратами инсулина [9, 12].
Дальнейшее изучение эффектов иНГЛТ-2 у пациентов с СД2 показало их дополнительное положительное влияние на сердечно-сосудистые и почечные исходы. По результатам мета-анализа трех крупных рандомизированных клинических исследований [DECLARE-TIMI-58 (дапаглифлозин), EMPA-REG OUTCOME (эмпаглифлозин) и CANVAS (канаглифлозин)] частоты сердечно-сосудистых и почечных исходов в объединенной популяции пациентов с СД2 (n=34322) установлено, что применение иНГЛТ-2 ассоциировано со снижением относительного риска значимых сердечно-сосудистых событий (нефатальный инсульт, нефатальный инфаркт, сердечно-сосудистая смерть) на 11%, госпитализации по поводу сердечной недостаточности (СН) на 31% и прогрессирования почечной недостаточности на 45%. При этом снижение риска развития значимых сердечно-сосудистых событий (МАСЕ) было достоверным только для пациентов с установленными атеросклеротическими сердечно-сосудистыми заболеваниями (АССЗ). Влияние же иНГЛТ-2 на частоту госпитализаций по поводу СН не зависело от наличия у пациентов АССЗ или предшествовавшей СН. Аналогично замедление прогрессирования хронической болезни почек (ХБП) не зависело от наличия у пациентов АССЗ и наблюдалось у пациентов с легким, умеренным и значительным снижением рСКФ [13].
Следует отметить, что в исследовании DECLARE-TIMI-58 (n=17160) в группе дапаглифлозина по сравнению с плацебо наблюдалось снижение частоты событий первичной конечной точки: сердечно-сосудистой смерти и/или госпитализации по поводу СН (ОР=0,83; 95% ДИ: 0,73–0,95; р=0,005) (рис. 1).
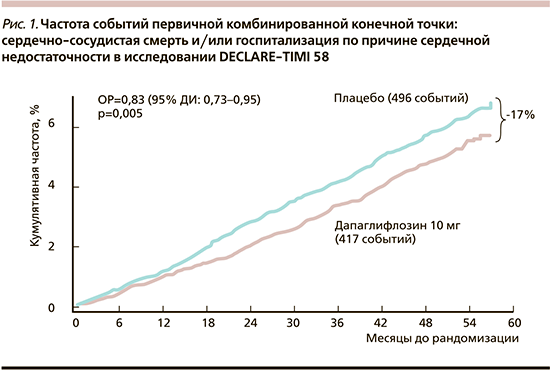
В отношении второй первичной конечной точки (МАСЕ) была установлена только тенденция к более низкой частоте сердечно-сосудистых событий в группе дапаглифлозина по сравнению с плацебо (ОР=0,93; 95% ДИ: 0,84–1,03; р=0,17), не имевшая статистической значимости. В исследовании DECLARE-TIMI-58 дапаглифлозин продемонстрировал значимое положительное влияние на прогрессирование ХБП у пациентов с СД2. Частота случаев вторичной почечной конечной точки (устойчивое снижение рСКФ на ≥40%, развитие терминальной стадии почечной недостаточности или смерть от почечной либо сердечно-сосудистой причины) в группе дапаглифлозина были значимо меньше, чем в группе плацебо (ОР=0,76; 95% ДИ: 0,67–0,87), что соответствует снижению относительного риска на 24%.
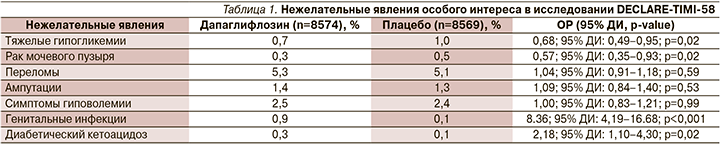
В данном исследовании был уточнен и подтвержден профиль безопасности дапаглифлозина. Так, была зарегистрирована достоверно меньшая частота серьезных нежелательных явлений по сравнению с плацебо (34,1 против 36,2%; ОР=0,91; 95% ДИ: 0,87–0,96; р<0,001) [14] (табл. 1).
По результатам исследования DECLARE-TIMI-58, в России в конце 2019 г. для дапаглифлозина было зарегистрировано новое показание в инструкции «сахарный диабет 2 типа у взрослых пациентов с установленным диагнозом ССЗ или двумя и более факторами сердечно-сосудистого риска для снижения риска госпитализации по поводу СН». К факторам риска относятся возраст у мужчин ≥55 лет или ≥ 60 лет у женщин и наличие не менее одного фактора риска (дислипидемия, артериальная гипертензия, курение) [12].
Дальнейшее изучение эффективности иНГЛТ-2 лежало за рамками их сахароснижающего действия и включало исследования частоты сердечно-сосудистых и почечных исходов в популяции пациентов как с СД2, так и без него. Первым препаратом, в отношении которого были запланированы и выполнены такие исследования, стал дапаглифлозин.
В 2019 г. закончилось исследование III фазы DAPA-HF, посвященное изучению эффектов дапаглифлозина у пациентов с установленным диагнозом СН со сниженной фракцией выброса (СНнФВ) вне зависимости от наличия СД2 (n=4744). В исследование включались лица с СНнФВ II–IV классов по NYHA (классификации Нью-Йоркской кардиологической ассоциации) и фракцией выброса ≤40%, имеющие повышенный уровень N-концевого натрийуретического пептида (NT-proBNP) в плазме. Пациентам назначался дапаглифлозин в дозе 10 мг 1 раз в сутки по сравнению с плацебо в дополнение к стандартной рекомендованной терапии СН. Медиана наблюдения пациентов в исследовании составила 18,2 месяца.
Частота событий первичной комбинированной конечной точки, которая включала госпитализацию либо обращение за неотложной медицинской помощью по поводу СН, либо сердечно-сосудистую смерть, была на 26% ниже в группе дапаглифлозина, чем в группе плацебо (ОР=0,74; 95% ДИ: 0,65–0,85; р<0,001) (рис. 2). Дапаглифлозин продемонстрировал преимущество в отношении каждого из трех компонентов первичной конечной точки. Число пациентов, которых нужно было пролечить дапаглифлозином для предотвращения одного события первичной конечной точки (NNT), составило 21 (95% ДИ: 15 – 38).
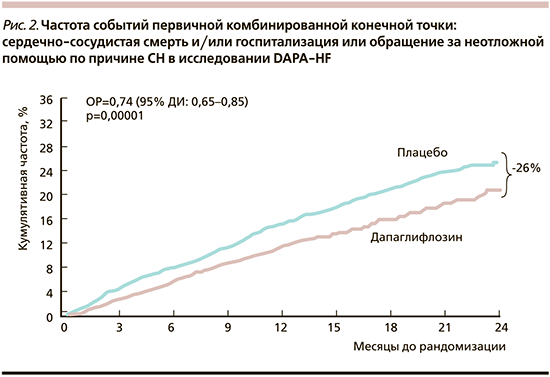
В группе дапаглифлозина также была ниже частота вторичной конечной точки, включившей госпитализации по поводу СН или сердечно-сосудистой смерти, – 16,1% по сравнению с 20,9% случаев в группе плацебо (ОР=0,75; 95% ДИ: 0,65–0,85; р<0,001).
Также хочется подчеркнуть, что в группе дапаглифлозина по сравнению с плацебо было на 17% меньше случаев общей смертности (ОР=0,83; 95% ДИ: 0,71–0,97).
Эффект дапаглифлозина в отношении событий первичной конечной точки ухудшения течения СН или сердечно-сосудистой смерти в целом оставался неизменным во всех заранее предусмотренных подгруппах, включая наличие или отсутствие СД2, пол, возраст, фракцию выброса левого желудочка, NT-proBNP, заданное значение рСКФ и др. (т.е. не зависел от наличия указанных факторов) [18, 24].
Была проанализирована частота серьезных нежелательных явлений.
В целом их частота была ниже в группе дапаглифлозина по сравнению с плацебо независимо от фактора наличия или отсутствия СД2 (для взаимодействия p=0,16)[19].
Таким образом, в исследовании DAPA-HF были получены доказательства того, что у пациентов с СНнФВ дапаглифлозин снижал риск событий ухудшения течения СН либо смерти от сердечно-сосудистых заболеваний, а также ослаблял симптомы СН. Влияние дапаглифлозина на достижение первичных и вторичных точек не зависело от наличия у пациентов СД2. В целом нежелательные явления встречались с сопоставимой частотой в группах дапаглифлозина и плацебо вне зависимости от наличия СД. В группе без СД2 не отмечалось случаев значимой гипогликемии или диабетического кетоацидоза [18, 19].
Мета-анализ двух крупных рандомизированных клинических исследований (DAPA-HF и EMPEROR-Reduced) продемонстрировал, что иНГЛТ-2 у пациентов с СНнФВ снижают смертность от всех причин на 13% (объединенное ОР=0,87; 95% ДИ: 0,77–0,98; p=0,018) и уменьшают сердечно-сосудистую смертность на 14% (ОР=0,86; 95% ДИ: 0,76–0,98; p=0, 027). Применение иНГЛТ-2 также сопровождалось снижением на 26% относительного риска сердечно-сосудистой смерти и первой госпитализации по причине СН (ОР=0,74; 95% ДИ: 0,68–0,82; p<0,0001), а также снижением на 25% совокупности повторных госпитализаций по поводу сердечной недостаточности или смерти от сердечно-сосудистых заболеваний (ОР=0,75, 95% ДИ: 0,68–0,84; p<0,0001). Риск комбинированной почечной конечной точки также был снижен на 38% (ОР=0,62; 95% ДИ: 0,43–0,90; p=0,013) [26].
По результатам исследования DAPA-HF для дапаглифлозина в 2020 г. в России было зарегистрировано показание, которое вывело данный препарат из разряда сахароснижающих и сделало его кардиопротективным средством для лечения пациентов с СНнФВ «Сердечная недостаточность (II–IV функциональные классы по классификации NYHA) со сниженной фракцией выброса у взрослых пациентов для снижения риска сердечно-сосудистой смерти и госпитализации по поводу сердечной недостаточности». Дапаглифлозин включен в отечественные клинические рекомендации по лечению пациентов с СН [12, 20].
Результаты исследований иНГЛТ-2 легли в основу обновлений рекомендаций по лечению пациентов с СД2, выпущенных рядом ведущих медицинских сообществ.
Так, в разделе 9 «Фармакологические подходы к лечению гипергликемии: стандарты медицинской помощи при диабете-2021» рекомендаций Американской диабетической ассоциации (ADA) для пациентов с установленным АССЗ или признаками высокого риска АССЗ (лица ≥55 лет со стенозом коронарной артерии, сонной артерии или артерии нижних конечностей >50% или гипертрофией левого желудочка), а также СН или ХБП рекомендовано использовать иНГЛТ-2 или арГПП-1 (с доказанной эффективностью в отношении сердечно-сосудистых заболеваний) как часть сахароснижающей терапии независимо от уровня HbA1c, использования метформина и с учетом факторов, специфичных для пациента. При этом уточняется, что для пациентов с СН с фракцией выброса левого желудочка <45% рекомендован класс иНГЛТ-2; для пациентов с диабетической ХБП и альбуминурией предпочтительно применение иНГЛТ-2 с доказанными преимуществами в отношении замедления прогрессирования ХБП либо иНГЛТ-2 с преимуществами для ХБП, доказанными в исследованиях сердечно-сосудистых исходов, либо арГПП-1 (при наличии противопоказаний к иНГЛТ-2 или их непереносимости) [15].
В рекомендациях по диабету, предиабету и сердечно-сосудистым заболеваниям 2019 г., разработанных объединенной группой Европейского общества кардиологов (ESC) и Европейской ассоциации по изучению диабета (EASD) «иНГЛТ-2 (эмпаглифлозин, канаглифлозин и дапаглифлозин), рекомендованы для снижения риска госпитализации по поводу СН у пациентов с СД (класс рекомендаций – 1, уровень доказательности – А)». Также иНГЛТ-2 выделены в разделе рекомендаций по профилактике и лечению ХБП у пациентов с СД: «лечение иНГЛТ-2 (эмпаглифлозин, канаглифлозин или дапаглифлозин) ассоциировано с более низким риском почечных исходов и рекомендовано при рСКФ от 30 до <90 мл/мин/1,73 м2» (класс рекомендаций – 1, уровень доказательности – В) [16].
В 2020 г. выпущено Руководство по клинической практике по лечению диабета при хроническом заболевании почек KDIGO-2020. В этом документе описан новый комплексный патогенетический подход к лечению пациентов с СД2, имевших ХБП, для снижения риска прогрессирования заболевания почек и ССЗ. Медикаментозная сахароснижающая терапия пациентов с СД2 и ХБП в качестве препаратов первой линии должна включать метформин и иНГЛТ-2, а также дополнительные медикаментозные средства, необходимые для контроля гликемии. Метформин и иНГЛТ-2 рекомендуется использовать при ХБП-С1–С3б. «Пациентам с СД2 и ХБП, имеющим рСКФ ≥30 мл/мин/1,73 м2, рекомендуется применение метформина (класс рекомендаций – 1, уровень доказательности – В)». «Рекомендуется применение иНГЛТ-2 пациентам с СД2 и ХБП, имеющим рСКФ ≥30 мл/мин/1,73 м2 (класс рекомендаций – 1, уровень доказательности – A)». Считается возможным добавлять иНГЛТ-2 к другим сахароснижающим препаратам пациентам, не достигшим целевых значений гликемического контроля, а также в случаях, когда целевые показатели гликемии достигнуты, но могут быть безопасно снижены. Третьим классом сахароснижающих препаратов в иерархии патогенетической терапии СД2 и ХБП являются арГПП-1: «Пациентам с СД2 и ХБП, которые не достигли индивидуальных гликемических целей, несмотря на использование метформина и иНГЛТ-2, или которые не могут использовать эти препараты, рекомендуем арГПП-1 длительного действия (класс рекомендаций – 1, уровень доказательности – В)» [17].
В отечественных Алгоритмах специализированной медицинской помощи больным сахарным диабетом 2021 г. (10-й выпуск) препараты классов иНГЛТ-2 и арГПП-1 выделены как приоритетные в сахароснижающей терапии пациентов с СД2 и указанием на высокий риск АССЗ, а также пациентов с ХБП (С1–С3а) для снижения риска ее прогрессирования. Больным хронической СН или с высоким риском ее развития рекомендуется использование в составе сахароснижающей терапии иНГЛТ-2. В частности, для пациентов с СД2 и ХБП сформулирована следующая рекомендация: «для пациентов с СД2 и ХБП-С1–3а рассмотреть возможность применения иНГЛТ-2 или арГПП-1, показавших снижение риска прогрессирования ХБП и развития кардиоваскулярных событий» [8].
В рамках продолжения научно-исследовательской программы изучения эффектов дапаглифлозина было проведено двойное слепое плацебо-контролируемое многоцентровое исследование III фазы DAPA-CKD по оценке долгосрочной эффективности и безопасности дапаглифлозина у пациентов с ХБП (с СД2 и без него), результаты которого были опубликованы в сентябре 2020 г. [21].
В исследование включались взрослые пациенты с СД2 или без него с рСКФ от 25 до 75 мл/мин/1,73 м2 и соотношением альбумина к креатинину в моче от 200 до 5000 мг/г, получаювшие ингибитор АПФ или блокатор ангиотензиновых рецепторов в стабильной дозе не менее 4 недель до скрининга.
В исследование включены 4304 пациента (386 центров из 21 страны мира), которые были рандомизированы в группу дапаглифлозина 10 мг 1 раз в сутки либо плацебо в соотношении 1:1. Длительность наблюдения составила 2,4 года. Средний возраст пациентов составил 61,8 года, на долю лиц мужского пола приходилось 66,9%; среднее значение индекса массы тела составило 29,5 кг/м2, среднее значение рСКФ – 43,1 мл/мин/1,73 м2. При этом большинство пациентов имели ХБП-С3б 44,1% и -С3а – 30,9% и в большинстве относились к категории альбуминурии А3 – 89,7% [21, 22].
В популяции исследования 67,5% пациентов имели диагноз СД2.
Безусловно исходные характеристики пациентов с СД2 и без него имели некоторые отличия, включая средний возраст, уровень HbA1c, распределение по группам рСКФ, а также этиологию ХБП. Среди пациентов с СД2 наиболее частой причиной ХБП звучала диабетическая нефропатия (86,4%), в группе пациентов без СД2 – хронический гломерулонефрит (42,8%) и ишемическая/гипертоническая нефропатия (34,8%). Диагноз подтвержден биопсией почки у 12,8% пациентов с СД2 и 35,8% пациентов без диабета [22, 23].
Наиболее распространенные сопутствующие заболевания в общей популяции: артериальная гипертония (95,7% пациентов), ожирение (44,5%) и ССЗ (37,4%), среди которых на долю СН приходилось 10,9%, инфарктов миокарда – 9,1%, инсультов – 6,9%. Пациенты с СД2 отличались более высокой распространенностью ожирения, артериальной гипертонии и ССЗ [22, 23].
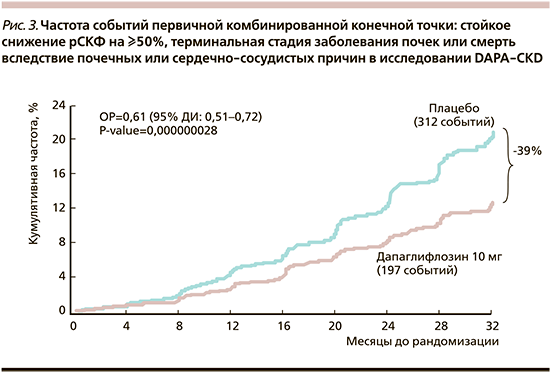
В группе дапаглифлозина была ниже частота событий первичной комбинированной конечной точки (включившей стойкое снижение рСКФ на ≥50%, терминальную стадию заболевания почек или смерть вследствие почечных или сердечно-сосудистых причин), чем в группе плацебо – 9,2% по сравнению с 14,5% (ОР=0,61; 95% ДИ: 0,51–0,72; р<0,001) (рис. 3). При этом превосходство дапаглифлозина распространялось на все компоненты первичной конечной точки. Число пациентов, которым было необходимо провести лечение за период исследования для предупреждения одного события первичной конечной точки (NNT), составило 19 (95% ДИ: 15–27) [21].
Положительный эффект дапаглифлозина в отношении более низкой частоты первичной конечной точки распространялся на все предварительно заданные подгруппы пациентов (возраст старше или младше 65 лет, пол, раса, географический регион, величина рСКФ ≥ 45 мл/мин/1,73 м2 или <45 мл/мин/1,73 м2, соотношение альбумина и креатинина в моче ≤1000 или >1000 мг/г, систолическое артериальное давление ≤130 или >130 мм рт.ст.). Также эффект дапаглифлозина сохранялся у больных СД2 и без него [21].
Дапаглифлозин продемонстрировал преимущество в отношении всех вторичных конечных точек. Отношение рисков для вторичной почечной комбинированной конечной точки (стойкое снижение рСКФ на ≥50%, терминальная стадия заболевания почек или смерть вследствие почечных причин) составило 0,56 (95% ДИ: 0,45–0,68; р<0,001); комбинированной вторичной конечной точки, включившей сердечно-сосудистую смерть или госпитализацию по поводу СН – 0,71 (95% ДИ: 0,55–0,92; р=0,009); конечной точки смерти от любой причины – 0,69 (95% ДИ: 0,53–0,88; р=0,004) [21].
Частота любых серьезных нежелательных явлений в исследовании DAPA-CKD в группе дапаглифлозина была существенно ниже (29,5% пациентов), чем в группе плацебо (33,9%; р=0,002). Среди нежелательных явлений, представляющих особый интерес, в отношении числа ампутаций, переломов и поражений почек – различия между группами дапаглифлозина и плацебо отсутствовали. События значимой гипогликемии чаще встречались в группе плацебо (1,3 против 0,7%, р=0,04). Ситуации гиповолемии чаще встречались в группе дапаглифлозина (5,9 по сравнению с 4,2% в группе плацебо; р=0,01). Диабетический кетоацидоз не встречался в группе дапаглифлозина, в то время как в группе плацебо он был зафиксирован у 2 пациентов. У лиц без СД2 случаев диабетического кетоацидоза или тяжелой гипогликемии не отмечено. В группе плацебо документирован один подтвержденный случай гангрены Фурнье [21].
В предварительно запланированнном подгрупповом анализе исследования DAPA-CKD проведена дополнительная оценка влияния диабетического статуса на эффект дапаглифлозина в достижении первичных и вторичных конечных точек. Снижение относительного риска для первичной комбинированной почечной конечной точки при применении дапаглифлозина наблюдалось у пациентов как с СД2 (ОР=0,64, 95% ДИ: 0,52–0,79), так и без диабета (ОР=0,50, 95% ДИ: 0,35–0,72); значение р для взаимодействия составило 0,24. Аналогичные результаты были получены для всех вторичных конечных точек (табл. 2) [23].
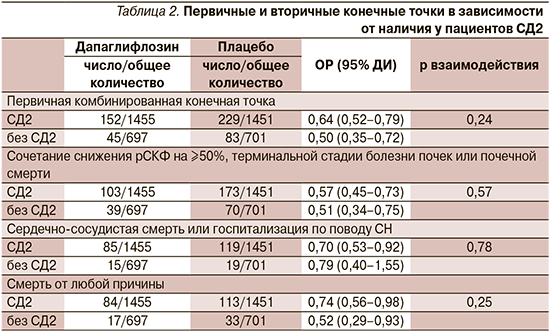
Доли пациентов в группах дапаглифлозина и плацебо, у которых развились серьезные нежелательные явления или был прекращен прием исследуемого препарата из-за нежелательных явлений, не различались между группами с СД2 и без него [23].
Заключение
По данным представленного исследования, у пациентов с ХБП (включая лиц с низкими значениями рСКФ до 25 мл/мин/1,73 м2), получавших дапаглифлозин, зарегистрировано статистически значимое снижение риска событий комбинированной почечной конечной точки, включившей стойкое ухудшение расчетной СКФ по крайней мере на 50%, терминальную стадию заболевания почек или смерть вследствие почечных или сердечно-сосудистых причин, по сравнению с плацебо. Кроме того, у получавших дапаглифлозин пациентов наблюдался более низкий риск госпитализаций по поводу СН и смерти вследствие сердечно-сосудистых причин, а также более высокая выживаемость. Эффекты дапаглифлозина не зависели от наличия у пациентов СД2, ССЗ, от уровня рСКФ, ОАКМ, а также от ряда других признаков. В исследовании подтвержден благоприятный профиль безопасности дапаглифлозина.
В конце апреля 2021 г. Управление по санитарному надзору за качеством пищевых продуктов и медикаментов США (FDA) одобрило дапаглифлозин для снижения риска почечных и сердечно-сосудистых событий у пациентов с ХБП, подверженных риску прогрессирования заболевания, независимо от того, страдают они СД или нет. Это делает дапаглифлозин первым иНГЛТ2, одобренным для лечения ХБП у взрослых пациентов независимо от диабетического статуса. Дапаглифлозину присвоен статус Fast Track («ускоренная процедура рассмотрения» препаратов для лечения серьезных заболеваний и решения неудовлетворенных медицинских потребностей), Breakthrough Therapy («прорывная терапия» – препараты для лечения серьезного состояния) и Priority Review («приоритетный статус рассмотрения» применяется для препаратов, спососбных значительно улучшить безопасность или эффективность лечения) [25].
В России в конце 2020 г. производителем дапаглифлозина компанией АстраЗенека была подана заявка на регистрацию нового показания к лечению ХБП у взрослых пациентов независимо от наличия СД2, одобрение которой ожидается в ближайшее время.



