Введение
Почечно-клеточный рак (ПКР) – гетерогенная группа злокачественных новообразований, которые развиваются из клеток проксимальных извитых канальцев почки [1]. В настоящее время выделяют 4 основных гистологических подтипа ПКР, для каждого из которых характерны специфичные молекулярно-генетические повреждения, определяющие потенциал злокачественности, метастазирования и чувствительности к лекарственному лечению [2, 4].
Ежегодно в мире регистрируют приблизительно 210–250 тыс. новых случаев заболевания ПКР, что составляет 2–3% в структуре злокачественных новообразований у взрослых [5]. В России среди опухолей мочеполовой системы ПКР занимает 2‑е место после злокачественных новообразований предстательной железы [6].
К моменту постановки диагноза у трети больных выявляют диссеминированную форму заболевания с отдаленными метастазами, среди которых первое место занимают метастазы в легких (50%), второе – в костях (30–40%) [1].
ПКР считается иммуногенной опухолью, что доказывается редким феноменом спонтанного регресса метастазов после проведения нефроэктомии и эффективностью цитокиновой терапии интерфероном-α и интрелейкином-2 [7].
Основой для разработки новых иммуноонкологических препаратов послужили результаты исследований, показавшие, что опухолевые клетки для «ускользания» от иммунологического надзора используют механизмы, в физиологических условиях необходимые для предотвращения развития аутоиммунной агрессии и повреждения собственных тканей [14]. Регуляция этого процесса осуществляется клеточными и молекулярными факторами, значительное место среди которых занимают ингибиторные рецепторы Т-клеток, т.н. контрольные точки иммунитета [15].
Блокирование ключевых регуляторных точек иммунного ответа – одно из самых многообещающих направлений научных исследований в лечении онкологических заболеваний.
Основные факторы распознавания опухоли клетками иммунной системы: ее антигенная характеристика, наличие опухолеспецифических и опухолеассоциированных антигенов, определяющих ее иммуногенность. Частота возникновения мутаций в опухолях почки высока, это может способствовать существенной антигенности данного вида рака и делает его крайне привлекательным для использования методов иммунотерапии. Регуляция активности Т-клеток обеспечивается различными костимуляторными и ингибирующими молекулами, находящимися на поверхности Т-лимфоцитов. Мишенью ингибиторов контрольных точек иммунного ответа служат рецепторы и связанные с ними регуляторные пути, влияющие на активность Т-лимфоцитов путем уменьшения ингибиторных сигналов, и как результат – активации Т-клеток для усиления противоопухолевой защиты.
Наиболее изученные из них CTLA-4 (cytotoxic T-lymphocyte associated protein 4 – CD152) [18] и PD-1 (рrogrammed cell death pathway 1) [16].
Ингибирующий рецептор CTLA-4 имеет сходную структуру с костимуляторным рецептором (CD28) на поверхности Т-клеток и является ключевым элементом в процессе активации Т-лимфоцитов. Для трансформации наивных Т-клеток в зрелые эффекторы необходим дополнительный неспецифический сигнал. Костимуляторами в данном случае выступают молекулы В7. Экспрессия CTLA-4 на Т-клетках усиливается в процессе их активации и начинает конкурировать с CD28 при взаимодействии с костимуляторными молекулями B7-1 и B7-2 на поверхности антигенпрезентирующих клеток. Таким образом, вместо усиления активации Т-клеток и их эффекторных функций взаимодействие B7:CTLA-4 ингибирует T-клеточную активацию преимущественно в лимфоидной ткани [8]. Ипилимумаб, моноклональное анти-CTLA4-антитело, блокирует взаимодействие B7:CTLA-4, таким образом сдвигая Т-клеточное равновесие в сторону повышения активности и эффекторной функции Т-лимфоцитов с последующим противоопухолевым действием [9].
Аналогично CTLA-4-рецептор PD-1 начинает экспрессироваться на активированных T-клетках. Взаимодействие PD-1 с лигандом приводит к ингибированию рецептора Т-лимфоцитов (TCR) и супрессии Т-клеточной эффекторной функции.
В то время как активация CTLA-4 приводит к подавлению активации Т-клеток в лимфоидной ткани, активность PD-1 проявляется главным образом в опухолевом микроокружении, где стимуляция данных рецепторов ограничивает Т-клеточный лизис опухолевых клеток (рис. 1). Гиперэкспрессия PD–L1 на опухолевых клетках указывает на то, что сигнальный путь PD-1 – это один из механизмов уклонения опухоли от иммунного ответа [17].

Применение ингибиторов контрольных точек иммунного ответа при мПКР
Появление в клинической практике современных иммуноонкологических препаратов изменило прогноз заболевания для многих пациентов с различными злокачественными новообразованиями, в т.ч. с ПКР.
Иммунотерапия при метастатическом раке почки в настоящее время – один из основных методов лекарственной терапии как в первой, так и последующих линиях лечения.
Первым зарегистрированным ингибитором контрольных точек иммунного ответа для мПКР является ниволумаб, представляющий собой человеческое моноклональное антитело к рецептору PD-1. Одобрение ниволумаба основано на результатах рандомизированного исследования III фазы CheckMate 025. В данное исследование был включен 821 пациент с мПКР.
У всех больных было зарегистрировано прогрессирование заболевания после таргетной терапии антиангиогенными препаратами [9]. Пациенты были рандомизированы в 2 группы, одна из которых получала ниволумаб (3 мг/кг в/в каждые 2 недели), вторая – эверолимус в дозе 10 мг внутрь ежедневно. Основным критерием эффективности была оценка общей выживаемости (ОВ). Кроме того, оценивались объективный ответ, время до прогрессирования и безопасность препарата.
Половина пациентов имели промежуточный прогноз по шкале MSKCC и 15% больных – плохой. Около 30% пациентов получили 2 линии предшествовавшей антиангиогенной терапии.
Ниволумаб продемонстрировал достоверное увеличение медианы ОВ на 6,4 месяца по сравнению с эверолимусом (26,0 против 19,7 месяца), не оказывая значимого влияния на выживаемость без прогрессирования, – ВБП (4,6 против 4,4 месяца) (рис. 2).
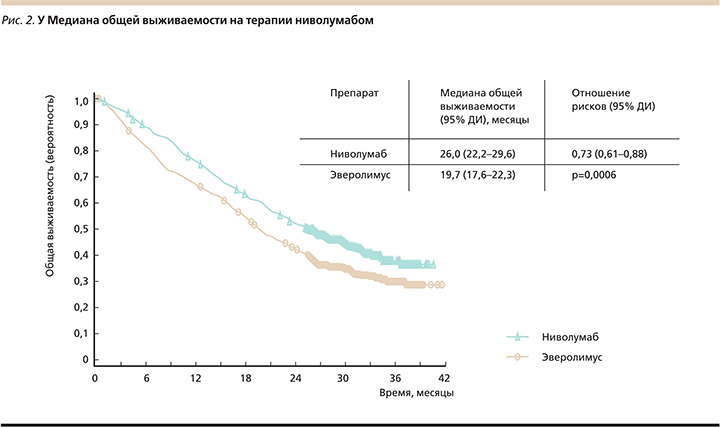
При проведении post-hoc-анализа у больных, не умерших от в течение первых 6 месяцев терапии и не имевших признаков прогрессирования заболевания, медиана ВБП составила 15,6 и 11,7 месяца в исследуемой и контрольной группах соответственно [19].
Общая частота ответов составила 26% для больных, получавших ниволумаб (по сравнению с 5% для принимавших эверолимус), при медиане длительности ответа 12,0 месяцев и медиане до наступления ответа 3,5 месяца. Продолжавшийся ответ на терапию ниволумабом имели 29% пациентов (по сравнению с 14% больных, получавших терапию эверолимусом). У большинства больных наблюдался ответ при первой оценке. Основной причиной прекращения лечения стало прогрессирование заболевания, после которого 44% больных смогли получить последующую терапию другими препаратами. Ниволумаб продемонстрировал преимущество ОВ над эверолимусом во всех группах прогноза вне зависимости от числа предшествовавших линий терапии и их длительности и при всех локализациях метастазов, включая метастазы в костях, печени и легких вне зависимости от их числа [20]. Интересен тот факт, что лечение оказалось также эффективным для пациентов с плохим прогнозом, т.е. для больных, для которых не существует препарата с доказанной эффективностью во 2-й линии терапии. Улучшение выживаемости не зависело от экспрессии PD-L1, что позволило назначать препарат ниволумаб без определения уровня данного маркера. При последующем проведении подгруппового анализа также было показано, что эффективность терапии ниволумабом не зависит от специфики препаратов, которые получали пациенты в предшествовавшие линии терапии (сунитиниб, пазопаниб, интерлейкин-2) [21].
Таким образом, ниволумаб продемонстрировал большую эффективность в сочетании с умеренным профилем токсичности по сравнению со стандартной терапией эверолимусом. Результаты этого и других исследований послужили основанием для разработки комбинаций. Новые моноклональные антитела (авелумаб, пембролизумаб и атезолизумаб) исследуются в комбинации с таргетными препаратами и бевацизумабом. В сравнительном исследовании атезолизумаб с бевацизумабом не продемонстрировали преимущества по сравнению с сунитинибом в общей когорте пациентов, но была отмечена положительная тенденция со стороны лиц с экспрессией PD-L1 [10]. Комбинации авелумаба и акситиниба [11], пембролизумаба и пазопаниба [12] в исследованиях I и I/II фаз соответственно продемонстрировали как высокую частоту ответов (более 55%), так и выраженную токсичность, что даже ограничило дальнейшее изучение второй комбинации.
В другом клиническом исследовании III фазы CheckMate 214 проводилось сравнение комбинации ниволумаба и ипилимумаба и стандартной терапии сунитинибом [13]. В исследование были включены пациенты с мПКР, относившиеся к группам промежуточного и неблагоприятного прогноза и ранее не получавшие лечения по поводу распространенного процесса [13].
Лечение в группе комбинированной терапии проведено по следующей схеме: ниволумаб 3 мг/кг, ипилимумаб 1 мг/кг каждые 3 недели 4 введения с последующим применением ниволумаба в дозе 3 мг/кг каждые 2 недели. Сунитиниб назначали в дозе 50 мг ежедневно в течение 4 недель с последующим перерывом 2 недели. Лечение продолжалось до прогрессирования заболевания или непереносимой токсичности. К основным критериям эффективности отнесены показатели ВБП, ОВ и частоты объективных ответов.
Медиана ВБП составила 11,5 месяца в группе ниволумаба и ипилимумаба (95% доверительный интервал [ДИ] – 8,71–15,51) и 8,38 месяца в группе сунитиниба (95% ДИ – 7,03–10,81). Частота объективных ответов в группе комбинации составила 42 против 26% в группе сунитиниба. Частота полного ответа – 9 против 1% соответственно. Побочные эффекты 3–4-й степеней в группе комбинации составили 43 против 63% в группе сунитиниба. Данные результаты позволили апробировать данную комбинацию иммунотерапевтических препаратов в качестве первой линии лечения больных мПКР, относившихся к группам промежуточного и неблагоприятного прогноза.
Таким образом, изучение комбинаций новых иммунных препаратов для больных мПКР представляется весьма актуальным. Возможно, совсем скоро новые комбинации появятся в ежедневной клинической практике.
Опыт применения ниволумаба
С 2015 г. по настоящее время в рамках программы расширенного доступа СА209254 на базе ФГБУ НМИЦ онкологии им. Н.Н. Петрова Минздрава РФ 10 пациентов с раком почки (8 мужчин, 2 женщины) получали иммунотерапию ниволумабом в качестве второй линии. Все больные имели светлоклеточный гистологический вариант опухоли и диссеминированный характер заболевания: 10 (100%) больных имели метастатическое поражение легких, 5 (50%) – метастазы в костях, 4 (40%) – в лимфатических узлах. У 2 (20%) пациентов метастазы были в головном мозге и надпочечниках. Средний возраст составил 61 год (47–71 год). Все больные получали ниволумаб в дозе 3 мг/кг 1 раз в 2 недели. Суммарно выполнено 206 введений препарата (от 3 до 47 введений, среднее число – 20,6).
Объективный ответ был достигнут 3 (30%) пациентами (один полный и два частичных регресса). Стабилизация была зарегистрирована у 5 (50%) пациентов. У 2 (20%) пациентов было установлено прогрессирование. Профиль токсичности был благоприятным и управляемым. Иммуноопосредованные побочные эффекты 3-й степени наблюдались у 1 (10%) больного в виде кожной токсичности. Из клинически значимых осложнений 2-й степени: пульмонит у 2 (20%) пациентов, мукозиты (глоссит и конъюнктивит) у 2 (20%), миозит и реактивный артрит у 1 (10%) больного. Осложнения 3-й степени были купированы длительным применением высоких доз преднизолона (1–2 мг/кг).
В настоящее время ниволумаб остается первым и единственным ингибитором PD-1, показавшим свою эффективность для больных метастатическим светлоклеточным раком почки в качестве второй линии лекарственной терапии. Полученные нами результаты подтверждают, что терапия ниволумабом обладает значимой клинической эффективностью, приемлемым и управляемым спектром токсичности.
Клинический случай
Больной В. 59 лет. Из анамнеза известно, что 28.03.2014 в зарубежной клинике ему было произведено оперативное вмешательство в объеме правосторонней нефрэктомии по поводу светлоклеточного рака почки. Прогрессирование заболевания отмечено с июля 2014 г. в виде появления метастазов в правом легком. Произведено оперативное вмешательство в объеме правосторонней метастазэктомии и химиоперфузии правого легкого.
По данным компьютерной томографии (КТ) органов грудной клетки и брюшной полости (от 19.03.2015), выявлено дальнейшее прогрессирование рака почки в виде множественных метастазов в легких и лимфатических узлах средостения. С 04.2015 начата первая линия лекарственной терапии (таргетная терапия пазопанибом в дозе 800 мг/сут). Лечение осложнилось возникновением ладонно-подошвенного синдрома 2-й ст., кожной токсичностью 2-й ст., артериальной гипертензией 2-й ст., гепатотоксичностью 2-й ст.
Максимальный эффект лечения – стабилизация заболевания в течение 9 месяцев.
По данным контрольного обследования (КТ органов грудной клетки и брюшной полости от 26.01.2016) зарегистрировано дальнейшее прогрессирование метастатического процесса в виде увеличения размеров и числа метастазов в легких и лимфатических узлах средостения. Больному было предложено участие в программе расширенного доступа к препарату ниволумаб. Обследован до начала иммунотерапии, выявлен субклинический гипотиреоз (повышение уровня тиреотропного гормона – ТТГ до 0,035 мЕД/л от 16.03.2016). Консультирован эндокринологом, назначена терапия тироксином (эутирокс по 25 мг/сут).
С 24.03.2016 начата терапия ниволумабом в дозе 3 мг/кг массы тела (суммарно 228 мг) с интервалом в 2 недели. В течение 3 месяцев лечение переносил без осложнений. С июня 2016 г. (после 6-го введения ниволумаба) отметил появление малоинтенсивных болей в мелких суставах кистей и стоп, ощущение «скованности» в указанных суставах. При осмотре определялись отек, деформация и ограничение объема движений в некоторых суставах кистей. Данное состояние расценено как осложнение терапии ниволумабом со стороны опорно-двигательной системы (артрит 1–2-й ст.), не требовавшее прерывания иммунной терапии. Рекомендован прием нестероидных противовоспалительных средств, малых доз глюкокортикостероидов (5–10 мг/сут преднизолона внутрь) с положительным клиническим эффектом.
По данным КТ органов грудной клетки и брюшной полости (от 22.07.2016), отмечено достижение частичного регресса (уменьшение размеров метастазов в обоих легких и внутригрудных лимфатических узлах). Клинически значимых отклонений со стороны лабораторных показателей не отмечено (сохранялось повышение уровня ТТГ 1-й ст., α-амилазы 1-й ст.).
С марта 2016 по декабрь 2017 г. больному выполнено 45 введений ниволумаба в стандартной дозе (3 мг/кг массы тела). Через 5 дней после 45-го введения препарата (20.12.2017) отмечено развитие клинически значимого осложнения в виде макулопапулярной кожной сыпи 3-й ст., локализующейся на спине, груди, верхних и нижних конечностях и сопровождающейся интенсивным зудом. По поводу дерматологической токсичности назначена терапия преднизолоном в дозе 1 мг/кг/сут (80 мг/сут), местная терапия (мази с глюкокортикостероидами, антибиотиками). Терапия преднизолоном в высокой дозе осложнилась развитием стероидного сахарного диабета (повышение уровня глюкозы сыворотки до 12 ммоль/л), потребовавшим назначения инсулинотерапии, кушингоидного синдрома, правосторонней пневмонии. К марту 2018 г. отмечено полное разрешение дерматологической токсичности, позволившее постепенно (в течение 4 недель) отменить пероральный прием преднизолона. Уровень глюкозы полностью нормализовался в мае 2018 г., терапия инсулином завершена.
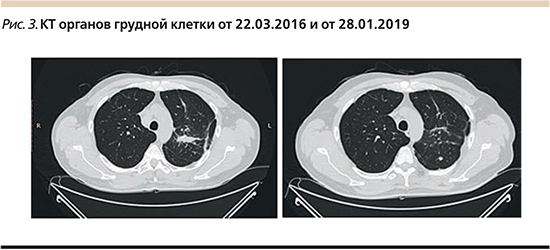
Согласно данным последней КТ органов грудной клетки и брюшной полости (январь 2019 г.), у пациента достигнут полный регресс метастазов в легких и лимфатических узлах средостения (рис. 3–5). Общая продолжительность эффекта – 32+ месяца.

Выводы
Увеличение ОВ больных в сочетании с удовлетворительной переносимостью терапии – основная цель лекарственного лечения распространенных злокачественных опухолей.
Появление иммуноонкологических препаратов в онкологии считается бесспорным достижением последнего десятилетия интенсивных научных изысканий и клинических исследований. Ингибиторы иммунных точек контроля демонстрируют достоверное преимущество прежде всего в ОВ больных диссеминированным раком почки как в первой (комбинация ниволумаба и ипилимумаба у пациентов с промежуточным и плохим прогнозом), так и во второй линии (монотерапия ниволумабом) лекарственной терапии. Многообещающие результаты демонстрируют продолжающиеся клинические испытания комбинаций ингибиторов иммунных точек контроля и таргетных препаратов, что, вероятно, в будущем послужит основанием для создания новых стандартов лечения.



