Введение
Результаты многочисленных исследований свидетельствуют о том, что прием фолиевой кислоты (ФК) – синтетического аналога витаминов группы В – во время беременности снижает риск возникновения у плода дефектов нервной трубки [31, 33]. Поэтому в настоящее время прием фолиевой кислоты в дозировке порядка 400 мг/день рекомендуют всем женщинам репродуктивного возраста в качестве меры первичной профилактики развития дефектов нервной трубки у будущего потомства [4].
ФК представляет собой стабильную синтетическую форму витаминов группы В. Ее можно использовать в виде таблеток или пищевых добавок, а кроме того, она содержится в витаминизированных продуктах питания. Метаболической активностью обладает не сама ФК, а ее производное тетрагидрофолат. Другой дериват – 5-метилтетрагидрофолат (5-МТГФ) – в норме обнаруживается в крови. Кроме того, именно 5-МТГФ содержится в продуктах питания. Он выпускается как пищевая добавка в чистом виде – [6S]-5-МТГФ – или в составе рацемической смеси [6RS]-5-МТГФ.
Фермент 5,10-метилентетрагидрофолатредуктаза (МТГФР) катализирует восстановление 5,10-метилентетрагидрофолата до 5-МТГФ, что необходимо для превращения гомоцистеина в метионин за счет присоединения углеродного остатка. Существует вариант гена МТГФР, в котором цитозин в положении 677 заменен на тимин (полиморфизм 677С → Т), в результате чего аминокислота аланин замещается валином. Среди европейцев с полиморфизмом данного гена 12 % составляют гомозиготы (ТТ), 43 % – гетерозиготы (СТ), а 45 % – аллели “дикого типа” (СС) [1, 10, 18, 24, 25]. В условиях in vitro активность фермента в случае генотипа ТТ снижена на 75 % по сравнению с аллелем “дикого типа” СС [9, 15], что ассоциировано с повышением сывороточного уровня гомоцистеина как следствие подавления синтеза 5-МТГФ (это становится особенно заметным при низком содержании ФК в крови) [1, 10, 17]. Более того, установлено, что вариант гена МТГФР 677С → Т служит генетическим фактором риска дефектов нервной трубки [35, 37], вызывая до 19 % случаев этой разновидности пороков развития [27, 32].
В ходе недавно завершенных клинических испытаний показано, что 5-МТГФ не менее эффективен по сравнению с ФК с точки зрения содержания фолатов в крови и эритроцитах; он также снижает уровень гомоцистеина как у клинически здоровых лиц, так и при наличии какой-либо патологии. Однако в большинстве случаев не принималось во внимание существование мутантного генотипа МТГФР (677С → Т) [12, 36]; генотип ТТ исключался [28] или же работа велась в небольших группах пациентов с гомозиготным генотипом, которым назначали разное лечение [7, 21, 22, 36, 38]. Таким образом, данные о влиянии [6S]-5-МТГФ и ФК на содержание фолатов в крови у лиц с генотипом ТТ ограничены. Кроме того, необходимо учитывать, что производные фолатов назначались в крайне высоких нефизиологических дозах, а иногда – в виде рацемической смеси 5-МТГФ.
В отличие от [6S]-5-МТГФ ФК должна быть восстановлена путем замены одного углеродного остатка. Этот процесс катализирует МТГФР. Затем продукт метаболизма в виде 5-МТГФ поступает в системный кровоток. Следовательно, в случае снижения активности МТГФР (что характерно для генотипа ТТ) эффект ФК относительно сывороточного уровня фолатов выражен в меньшей степени по сравнению с [6S]-5-МТГФ.
Цель настоящего исследования включает две задачи. Первая заключается в том, чтобы сравнить особенности фармакокинетики при использовании физиологической однократной пероральной дозы [6S]-5-МТГФ или ФК женщинами репродуктивного возраста с мутацией гена МТГФР 677С → Т по гомозиготному (ТТ) или “дикому” (СС) типу. Вторая задача – изучить генетические различия. Из фармакокинетических параметров оценивали профиль концентрации в зависимости от времени (площадь под кривой/AUC концентрации фолатов в плазме крови относительно времени), максимальное содержание фолатов (Сmax) и время, необходимое для достижения максимального общего сывороточного уровня фолатов (tmax). Кроме того, проанализированы степень кратковременной абсорбции и особенности начального метаболизма ФК и [6S]-5-МТГФ in vivo путем определения концентрации ФК, 5-МТГФ, тетрагидрофолата (ТГФ) и 5,10-метенилтетрагидрофолата (5,10-СНТГФ).
Методы
Пациенты
Клинически здоровые женщины с мутацией гена МТГФР 677С → Т по генотипу ТТ или СС были отобраны из баз данных предыдущих исследований, проведенных Институтом питания (Университет Бонна, Германия). Для цели настоящего исследования подходили женщины репродуктивного возраста с индексом массы тела 17–25 кг/м², нормальными показателями общего и биохимического анализов крови, соответствующим содержанием фолатов (> 6,8 нМ в плазме крови и > 317 нМ в эритроцитах) и витамина В12 (> 110 пМ в плазме крови). Кроме того, все пациентки пользовались надежными контрацептивными средствами. Основными критериями исключения стали наличие органических или психических заболеваний, применение лекарственных средств, влияющих на метаболизм фолатов (метотрексат, сульфасалазин, салициловая кислота, противоэпилептические препараты), беременность или период кормления грудью, злоупотребление алкоголем или медикаментами. Испытуемых попросили придерживаться стандартного режима питания в течение 4 недель до начала исследования и на протяжении всего срока его проведения, избегая при этом дополнительного приема витаминов или употребления пищи, обогащенной фолатами. Дизайн исследования был одобрен Этическим комитетом Медицинской ассоциации Гамбурга (Германия). Оно выполнялось в соответствии с принципами Хельсинкской декларации. Все испытуемые давали добровольное информированное согласие в письменном виде.
Дизайн
В соответствии с дизайном проведено рандомизированное двойное слепом перекрестное исследование (рис. 1). Клиническая часть заняла 3 дня (скрининг, день I и день II), причем скрининг выполнялся за 12 дней до первого дня исследования. День I и день II были разделены промежутком в 6 суток (период отмены). В качестве лечения испытуемым назначали таблетки немедленного высвобождения, покрытые пленчатой оболочкой, которые содержали 400 мкг ФК или 416 мкг [6S]-5-МТГФ. Женщин подвергли рандомизации для выбора одной из схем лечения – [6S]-5-МТГФ в день I и ФК в день II или ФК в день I и [6S]-5-МТГФ в день II. Рандомизацию стратифицировали в соответствии с полиморфизмом гена МТГФР 677С → Т, чтобы обеспечить одинаковое распределение генотипов ТТ и СС в обеих группах лечения. Утром I и II дней исследования у пациенток брали кровь натощак (после 12-часового перерыва в приеме пищи). Сразу после взятия анализа крови испытуемые принимали ФК или [6S]-5-МТГФ в однократной дозировке и запивали таблетку 200 мл воды. Затем кровь повторно брали на анализ в течение 8 часов после приема таблетки (через 30, 60, 90, 120, 180, 240, 360 и 480 минут). На протяжении дней исследования женщины соблюдали специальную диету в соответствии с результатами исследований, посвященных изучению биодоступности ФК/фолатов [29]. В эту диету был включен специальный напиток, не содержащий фолатов, который приготавливали путем растворения 85 г порошка в 240 мл воды (437 ккал на порцию). Этот напиток представляет собой коммерческую пищевую добавку (Scandishake Mix Vanille). Кроме того, дополнительно разрешалось выпивать 200 мл минеральной воды или черного кофе. Мониторинг переносимости лечения и его безопасность контролировали по специальным анкетам, которые заполнялись испытуемыми в I и II дни исследования, а также по результатам стандартных лабораторных анализов, которые выполнялись на II день исследования.
Образцы крови
Забор крови для проведения фармакокинетических исследований осуществляли из срединной локтевой вены в пробирки с гепариновым покрытием. Кровь центрифугировали в течение 15 минут (3000 × g, 10 минут). Отделившуюся плазму крови хранили при -80 °С в течение 3 месяцев. Для изучения содержания в крови производных фолатов (ФК, 5-МТГФ, ТГФ и 5,10-СНТГФ) кровь собирали в пробирки, покрытые ЭДТА, центрифугировали при 4 °С в течение 15 минут (1000 × g, 10 минут) и хранили при -80 °С.
Анализы
Общий уровень фолатов в крови оценивали с помощью иммунологического исследования на анализаторе Immulite 2000 (вариабельность в пределах постановки < 5,4 %; вариабельность между постановками < 8,3 %). Анализ проводился в два цикла. Вначале образец крови с меченной лигандом ФК обрабатывали дитиотреитолом, а затем – цианидом калия. Далее образец крови переносили в пробирку с полистироловыми шариками, которые содержали белок, связывающий фолаты, и антителами к нему. В ходе инкубации продолжительностью 30 минут за счет функционирования механизма конкурентного связывания взаимодействие между ФК и связывающим ее белком разрушалось, ФК высвобождалась, а белок ассоциировал с меченной лигандом ФК. После промывки антилиганды, меченные щелочной фосфатазой, взаимодействовали с меченными лигандами фолатами, которые связались с шариками в процессе первой инкубации. Свободные ферментные конъюгаты удаляли путем центрифугирования. Затем добавляли субстрат, и процедура анализа продолжалась по механизму типичной иммунной реакции. Уровень фолатов в плазме крови определяли после разведения вручную всех образцов крови в специальном растворителе (1 : 5). Чтобы избежать вариабельности в пределах постановки, все образцы от всех субъектов анализировали в один прием. Стандартные лабораторные исследования выполняли непосредственно после забора крови из вены на этапе скрининга и в конце второго дня исследования в центральной лаборатории университетской больницы Бонна (Германия). Определение уровней ФК, 5-МТГФ, ТГФ и 5,10-СНТГФ осуществляли в Медицинском центре Свободного Университета Амстердама (Голландия). В Амстердам образцы плазмы крови в замороженном виде доставлял курьер. Уровни различных производных фолатов оценивали посредством жидкостной хроматографии и тандемной масс-спектрометрии (для 5-МТГФ вариабельность в пределах постановки < 3,8 %, вариабельность между постановками < 1,7 %; для фолатов вариабельность в пределах постановки < 3,2 %, вариабельность между постановками < 7,1 %) [19]. Если описывать вкратце, то вначале к образцам плазмы крови добавляли [13С5]-5-МТГФ в качестве внутреннего стандарта. Далее сыворотку очищали на аффинных колонках с фолат-связывающим белком с последующей концентрацией. Жидкостную хроматографию и тандемную масс-спектрометрию проводили на тройном четырехполюсном тандемном масс-спектрометре API 3000 в положительном режиме, а затем делали мониторинг фрагментов-предшественников конкретных производных фолатов. Каждый образец крови исследовали дважды.
Статистическая обработка
Исследование носило характер пилотного. Представлялось возможным оценить только объем выборки, т. к. данных о содержании [6S]-5-МТГФ у клинически здоровых лиц с генотипом ТТ было недостаточно. На основании данных Prinz-Langenohl и соавт. [30], полученных в когорте гетерозиготных женщин с мутацией гена МТГФР 677С → Т, было установлено, что 16 человек с генотипом ТТ (при условии их соответствия критериям включения) достаточно для сравнения фармакокинетики [6S]-5-МТГФ и ФК. Для изучения возможного влияния генетических факторов на фармакокинетику этих витаминных добавок в исследование были дополнительно включены еще 8 женщин с генотипом СС. Полученные результаты отображались в виде среднего (n – количество испытуемых) ± SD. Анализ фармакокинетических параметров осуществляли в парных выборках по t-критерию, что позволило оценить эффект ФК и [6S]-5-МТГФ в отношении общего уровня фолатов в плазме крови. Средние AUC для каждого вида лечения представлены с 95 % доверительным интервалом (ДИ). Вычисление t-критерия осуществлялось с частотой двусторонней ошибки I рода 5 %. Определяя AUC для каждого отдельно взятого испытуемого и способа лечения (по правилам трапезоидных чисел), подсчитывали разницу относительно исходной величины (0 минут) в 9 временных точках. Значения AUC представлены в виде нМ с поправкой на время. Чтобы проанализировать относительную биодоступность каждого из использованных препаратов, величины AUC отражали в процентах. Анализ чувствительности предполагал оценку ковариации AUC (ANCOVA), что позволяло говорить о той или иной эффективности лечения. При описании корреляции для каждого пациента в качестве ковариации рассматривали последовательности лечения, варианты генотипов и сывороточные уровни фолатов до начала лечения. Конечные описательные результаты получены для общих сывороточных уровней фолатов, ФК, 5-МТГФ, ТГФ и 5,10-СНТГФ.
Статический анализ выполнялся с применением программы SAS, версия 9.1.3 (SAS Inc., Гейдельберг, Германия).
Материалы
[6S]-5-МТГФ (Метафолин – кальциевая соль [6S]-5-МТГФ) произведен компанией Merck Selbstmedikation GmbH (Дармштадт, Германия). Диетический напиток Scandishake Mix Vanille произведен компанией Nutricia Nahrungsmittel GmbH&CoKG (Вена, Австрия). Пробирки, покрытые гепарином и ЭДТА, произведены компанией Sarstedt (Нюмбрехт, Германия). Наборы для анализатора Immulite 2000 произведены компанией Diagnostic Products Corporation Biermann GmbH (Бад-Наухайм, Германия), тандемный масс-спектрометр API 3000 – компанией Applied Biosystem (Фостер-Сити, США).
Результаты
Характеристики испытуемых
Для скрининга была отобрана 31 женщина. Семь человек не удовлетворяли критериям включения и были отсеяны. Оставшиеся 24 испытуемые (ТТ = 16, СС = 8) были рандомизированы и участвовали в исследовании вплоть до его завершения. По этим женщинам был получен полный набор данных. Клинические характеристики пациенток представлены в табл. 1. По таким параметрам, как сывороточный уровень витамина В12, общая концентрация фолатов в плазме крови и концентрация фолатов в эритроцитах, рост, вес, индекс массы тела и основные показатели состояния организма, между генотипами и последовательностями лечения не было найдено никаких статистически достоверных различий. Поскольку одним из критериев включения было содержание фолатов в крови > 6,8 нМ и в эритроцитах > 317 нМ, в исследуемой когорте уровень фолатов в целом был высоким.
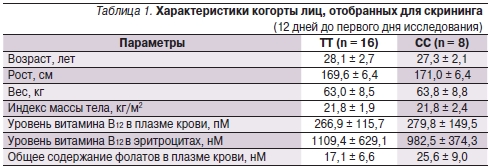
Примечание. Между группами не выявлено никаких статистически достоверных различий (по результатам одномерного анализа ANOVA).
Терапия переносилась добровольцами хорошо, никаких побочных явлений в процессе лечения не наблюдалось.
Общее содержание фолатов в плазме крови
На рис. 2 представлены средние общие сывороточные концентрации фолатов у лиц с генотипом ТТ до и после приема дозы лекарства. Средние абсолютные значения изменений уровня фолатов в крови относительно исходной величины (0 минут) оказались выше в группе принимавших [6S]-5-МТГФ по сравнению с группой принимавших ФК в течение всего периода наблюдений (480 минут). Как следует из рис. 2, степень всасывания [6S]-5-МТГФ и ФК отличается, а скорость выведения – нет. Аналогичная картина наблюдалась и у испытуемых с генотипом СС (рис. 3).
Фармакокинетические переменные
В табл. 2 отражены результаты оценки фармакокинетических показателей. В группе лиц с генотипом ТТ средняя AUC и Cmax для общей концентрации фолатов в крови оказались статистически достоверно выше (в 2 раза) после приема [6S]-5-МТГФ по сравнению с ФК (p < 0,0001). Среднее tmax было статистически достоверно меньше для [6S]-5-МТГФ по сравнению с ФК. Аналогичная картина наблюдалась и среди испытуемых с генотипом СС (табл. 2). Это было справедливо как для средней AUC и Cmax (p < 0,005), так и для tmax (p < 0,05).
Примечание. Данные представлены как арифметическое среднее ± СО с 95 % ДИ в скобках.
Для оценки различий между переменными использован t-критерий Стьюдента.
* Статистически достоверные отличия для tmax на фоне приема ФК в случае генотипа СС, р = 0,0217.
Статистически достоверные различия по фармакокинетике между генотипами ТТ и СС были выявлены только для tmax на фоне применения ФК (среднее tmax оказалось выше в группе ТТ по сравнению с группой СС).
Вне зависимости от генотипа [6S]-5-МТГФ в однократной дозировке обладает большей, чем ФК в аналогичной дозировке биодоступностью. Об этом можно судить по соотношениям значений AUC (для ТТ 200,95 % при 95 % ДИ 169,61–232,3 %; для СС 159,2 % при 95 ДИ 126,54–191,87 %).
Анализ ANCOVA не выявил никаких статистически достоверных корреляций между последовательностями лечения, генотипом или содержанием фолатов в крови до приема лекарственного препарата, с одной стороны, и сывороточным уровнем фолатов – с другой, хотя присутствовал статистически достоверный эффект от лечения (p < 0,0001).
Производные фолатов в плазме крови
Во всех случаях доминирующим производным фолатов был 5-МТГФ. У 5 женщин также обнаружился 5,10-СНТГФ в следовых количествах (≤ 3 нМ). У 2 человек выявлен ТГФ (≤ 4 нМ). На фоне приема ФК этот дериват определен у 18 из 24 человек (13 испытуемых с генотипом ТТ и 5 – с генотипом СС). Содержание ФК в плазме крови достигало пика через 90–120 минут после ее применения в таблетках, составив 14,3 ± 6,1 нМ. У 2 человек наблюдался дополнительный подъем уровня ФК в плазме крови, что было результатом использования [6S]-5-МТГФ (1 человек с генотипом ТТ, максимальная концентрация – 21,4 нМ; 1 человек с генотипом СС, максимальная концентрация – 6,1 нМ).
В анализах крови, взятых натощак в первый день исследования, ФК не была обнаружена ни у одной из женщин. Это свидетельствует о том, что все женщины соблюдали протокол лечения, поскольку прием пищи, обогащенной ФК, или пищевых добавок с ФК был прекращен за 4 недели до начала терапии.
Обсуждение
Полученные в ходе настоящего исследования данные свидетельствуют о том, что при назначении на непродолжительный срок в физиологической дозировке [6S]-5-МТГФ в большей степени, чем ФК, способствует повышению уровня фолатов в плазме крови, причем вне зависимости от генотипа мутации 677СА → Т гена МТГФР. Это утверждение можно сделать, сопоставив величины AUC: для [6S]-5-МТГФ она статистически достоверно выше по сравнению с ФК, т. е. [6S]-5-МТГФ обладает большей относительной биодоступностью. Между подгруппами лиц с различным генотипом мутации гена МТГФР не было выявлено никаких статически достоверных различий по фармакокинетическим параметрам, за исключением tmax, которое оказалось достоверно больше для генотипа ТТ (по сравнению с СС) на фоне приема ФК.
Представленные нами данные отличаются от результатов аналогичных исследований, поскольку в качестве испытуемых выступали молодые клинически здоровые женщины с генотипом ТТ, а для лечения использовались ФК и ее природная фолатная форма [6S]-5-МТГФ в биологически эквимолярных количествах и физиологически низкой дозировке.
Willems и соавт. (2004) показали [38], что у пожилых лиц с сердечно-сосудистыми заболеваниями вне зависимости от генотипа (ТТ или СС) биодоступность 5-МТГФ выше по сравнению с ФК. Но в этом исследовании в качестве лечения использовали рацемическую смесь [6RS]-5-МТГФ в большой (5 мг) дозировке. Кроме того, слабо учитывался такой фактор, как питание, что имеет принципиальное значение, поскольку [6R]-изомер, как считается, биологически неактивен. Наконец нельзя исключать и возможности побочных эффектов, ассоциированных с накоплением [6R]-изомера [23, 38]. Lamers и соавт. [20], Venn и соавт. [36] и Fohr и соавт. [7] также вели работы по изучению влияния генотипа МТГФР на содержание фолатов в плазме крови и эритроцитах/концентрацию гомоцистеина, назначая 5-МТГФ с ФК. Однако после рандомизации со стратификацией по генотипу оказалось, что численность когорты лиц с генотипом ТТ крайне мала. Вследствие этого оказалось невозможным сделать какие-то выводы по данной группе испытуемых.
В отличие от Pentieva и соавт. [28] нам удалось продемонстрировать, что при наличии генотипа СС биодоступность [6S]-5-МТГФ в ближайшем периоде больше по сравнению с ФК. Разница заключается в том, что в исследовании Pentieva и соавт. (2004) применяли принцип предварительного насыщения ФК, что могло повлиять на функциональную активность фолат-связывающего белка (об этом свидетельствуют данные Houghton и соавт., 2009).
В нашем исследовании было продемонстрировано, что вне зависимости от генотипа содержание фолатов в плазме крови достигает гораздо большего пикового значения и за более короткое время, если испытуемые принимали [6S]-5-МТГФ, а не ФК. Более того, tmax оказалось больше у лиц с генотипом ТТ по сравнению с генотипом СС на фоне использования ФК. Это может быть связано с различиями в метаболизме этих форм витамина, специфичными для конкретного генотипа. [6S]-5-МТГФ представляет собой биологически активное производное фолатов и может накапливаться в организме. Следовательно, прием [6S]-5-МТГФ может спровоцировать изменение сывороточной концентрации фолатов напрямую без эффекта первого прохождения или опосредованно – путем замещения печеночного 5-МТГФ. Равно как и [6S]-5-МТГФ, ФК в каких-то количествах может поступать в системный кровоток, не претерпевая биотрансформации в печени. Неметаболизированную ФК в крови удалось обнаружить только в ходе нашего исследования. Однако подавляющая часть ФК при приеме per os поглощается клетками слизистых оболочек и гепатоцитами, превращаясь в 5-МТГФ, который напрямую или опосредованно способствует повышению сывороточного уровня фолатов. Таким образом, пролонгирование tmax у лиц с генотипом ТТ на фоне использования ФК по крайней мере частично объясняется ослаблением активности МТГФР.
Методологический подход, использованный в настоящем исследовании, описывает только изменение общей концентрации фолатов в плазме крови и их клиренс на фоне использования [6S]-5-МТГФ и ФК. Остается непонятным, связано ли повышение уровня фолатов непосредственно с пероральным приемом препарата, или обусловлено перераспределением в тканях, о чем неоднократно заявляли другие ученые [39]. Более подробное изучение вариаций уровня фолатов в плазме крови и механизмов биотрансформации [6S]-5-МТГФ и ФК у лиц с различными генотипами предполагает исследования с применением меченых фолатов/ФК. Кроме того, важная задача дальнейших исследований – анализ возможных генетических ассоциаций на основании показателя секреции ФК и [6S]-5-МТГФ с мочой.
В отличие от других авторов [3, 11, 17, 26] в процессе скрининга мы не нашли никаких отличий в содержании фолатов в плазме крови и эритроцитах вне зависимости от генотипа. Возможно, это связано с тем, что, согласно критериям включения, женщины с низким фолатным статусом не допускались к участию в исследовании.
В ходе данного эксперимента мы выяснили, что после приема ФК этот метаболит появляется в крови практически во всех случаях (у 18 человек из 24), но лишь изредка – при использовании [6S]-5-МТГФ (у 2 человек из 24). С учетом этого можно предполагать, что [6S]-5-МТГФ в незначительной степени замещает принятую ранее (за неделю или месяц) ФК, которая даже по прошествии времени остается прочно связанной с печеночными фолат-связывающими белками. Действительно, максимальная пиковая концентрация ФК (21,4 нМ) на фоне приема [6S]-5-МТГФ была зафиксирована у женщины, которой в I день исследования назначили ФК и во II – [6S]-5-МТГФ.
Результаты других экспериментов свидетельствуют о том, что после перорального использования ФК даже в небольшой дозировке она обнаруживается в неметаболизированном виде в системном кровотоке [16] и грудном молоке [13]. Проведя популяционный анализ среди американок, употреблявших и не употреблявших продукты питания (зерновые), обогащенные в небольшом количестве ФК, Kalmbach и соавт. [14] сообщили о повышении уровня ФК в крови. Появление неметаболизированной ФК в кровотоке скорее всего связано с нарушением метаболизма, транспорта и функций природных фолатов в организме человека [33]. Этот симптом может служить проявлением скрытого дефицита витамина В12, который предрасполагает к необратимым повреждениям нервных структур [16]. Также обсуждается возможная роль ФК в подавлении цитотоксической активности естественных киллеров [34].
Таким образом, при использовании кратковременного протокола с эквимолярными дозами [6S]-5-МТГФ в большей степени, чем ФК, повышает общее содержание фолатов в крови вне зависимости от генотипа (ТТ или СС) мутации 677С → Т гена МТГФР. Это подтверждают результаты оценки фармакокинетических параметров, в частности AUC, у лиц с обоими разновидностями генотипов. Различия по tmax после приема ФК у лиц с генотипами ТТ и СС можно объяснить ослаблением активности МТГФР. По нашим наблюдениям, ФК часто появляется в плазме крови в неметаболизированном виде после ее добавления к лечению и лишь изредка после добавления [6S]-5-МТГФ. Поскольку о наличии серьезных побочных эффектов у [6S]-5-МТГФ ничего неизвестно, препараты на основе этой природной биологически активной формы фолатов могут выступать в качестве альтернативы добавкам с ФК. Возможность обогащения продуктов питания [6S]-5-МТГФ требует более углубленного изучения.


