Введение
Рак желудка (РЖ) является пятым по частоте встречаемости и четвертым по частоте смертности в структуре онкологических заболеваний [1]. Как правило, треть пациентов на момент постановки диагноза имеют отдаленные метастазы [1] или не подлежат хирургическому лечению. Несмотря на многообещающие результаты терапии, эффективной в отношении молекулярно-генетических альтераций при РЖ, число пациентов, которые в реальной клинической практике получают это лечение, невелико, что в большей степени обусловлено гетерогенностью опухоли [2]. Таким образом, в настоящее время системная химиотерапия (ХТ) остается основным методом лечения пациентов с диссеминированным РЖ и кардиоэзофагеального перехода (дРЖ/КЭП). Стандартным подходом к лечению больных дРЖ/КЭП является применение двойных комбинаций с включением препаратов платины и фторпиримидинов. Оксалиплатин по эффективности не уступает цисплатину и является предпочтительной опцией в большинстве схем лечения за счет снижения токсичности [3]. Схожую эффективность показала комбинация 5-фторурацила(5-ФУ) с иринотеканом (FOLFIRI), которая может служить альтернативой для пациентов с противопоказаниями к назначению платиновых агентов [4]. Прогноз пациентов с дРЖ/КЭП остается крайне неблагоприятным, медиана общей выживаемости (мОВ), по данным клинических исследований, редко достигает 1 года, а медиана выживаемости без прогрессирования болезни (мВБП), как правило, не превышает 6 месяцев [5–10]. Так, например, для наиболее популярного режима первой линии при дРЖ/КЭП mFOLFOX6 в исследовании METGastric показатели мВБП и мОВ составляли 6,8 и 11,3 месяца соответственно [9].
Опубликованы данные об улучшении показателей выживаемости при применении трехкомпонентных режимов ХТ, т.н. триплетов. Согласно результатам рандомизированного многоцентрового исследования III фазы V-325 по сравнению со стандартным дуплетом цисплатина с 5-ФУ (CF) трехкомпонентная комбинация доцетаксела, цисплатина и 5-ФУ (DCF) в первой линии лечения статистически значимо повысила эффективность лечения: медиана времени до прогрессирования составила 5,6 и 3,7 (p=0,0004), мОВ – 9,2 и 8,6 месяца (p=0,02) для триплета и дуплета соответственно [11]. Замена цисплатина на оксалиплатин не привела к снижению эффективности триплета по сравнению с дуплетом. Так, в исследовании II фазы при использовании трехкомпонентного режима TEF (доцетаксел, оксалиплатин и 5-ФУ) получено увеличение показателей выживаемости по сравнению с режимом TE (доцетаксел, оксалиплатин): мВБП – 7,66 (95% доверительный интервал [ДИ]: 6,97–9,40) и 4,50 месяца (95% ДИ: 3,68–5,32), мОВ – 14,59 (95% ДИ: 11,70–21,78) и 8,97 месяца (95% ДИ: 7,79–10,87) [12]. Триплеты, как правило, назначают сохранным пациентам при удовлетворительном общем статусе в связи с высокой токсичностью. С целью улучшения переносимости комбинации DCF разработана его модификация mDCF.
В исследовании II фазы M.A. Shah et al. проведено сравнение оригинальной комбинации DCF и ее модификации mDCF в первой линии лечения больных дРЖ/КЭП: мОВ при ХТ в режиме mDCF была статистически незначимо выше по сравнению с режимом DCF (18,8 против 12,6 месяца) при меньшей токсичности и лучшей переносимости [13]. Режим FOLFIRINOX (оксалиплатин, иринотекан, 5-ФУ), показавший высокую активность при некоторых видах рака желудочно-кишечного тракта, также исследовался в качестве первой линии ХТ дРЖ/КЭП в рамках нерандомизированного исследования II фазы H. Park et al.: мВБП составила 8,4 месяца, мОВ достигла 15,5 месяца [14]. Результаты данного исследования и ряда других небольших исследований II фазы комбинации FOLFIRINOX в первой линии лечения больных дРЖ/КЭР свидетельствовали о ее высокой эффективности при контролируемой токсичности [15–19].
Таким образом, в настоящее время нет исследований III фазы, убедительно показавших преимущество триплетов по сравнению со стандартными двойными комбинациями. Для ответа на данный вопрос в ФГБУ «Национальный медицинский исследовательский центр онкологии им. Н.Н. Блохина» Минздрава РФ в ноябре 2019 г. инициировано проспективное рандомизированное исследование III фазы, целью которого было изучение эффективности и токсичности режима FOLFIRINOX по сравнению с дуплетом mFOLFOX6 (ClinicalTrials.gov identifier: NCT04442984) в качестве первой линии терапии больных диссеминированной аденокарциномой желудка и КЭП. Профиль токсичности и тяжесть нежелательных явлений (НЯ) являются важным аспектом оценки при исследовании какого-либо режима ХТ [20–23]. В настоящей статье мы представляем данные по промежуточной оценке переносимости и токсичности.
Материал и методы
В исследование включали больных, имевших морфологически подтвержденную местнораспространенную нерезектабельную (T4b) или диссеминированную (M1) аденокарциному желудка или пищеводно-желудочного перехода (КЭП). Испытание проводилось в соответствии с принципами надлежащей клинической практики, законодательством Российской Федерации и Хельсинкской декларацией всемирной медицинской ассоциации в редакции от 2013 г. Методика и результаты исследования оценены согласно стандартам CONSORT2010 [24, 25].
Все пациенты имели гистологически подтвержденную нерезектабельную местнораспространенную или метастатическую аденокарциному желудка или желудочно-пищеводного перехода, не получали лекарственной терапии по поводу нерезектабельного или диссеминированного процесса и имели по крайней мере одно оцениваемое поражение в соответствии с критериями RECIST v.1.1 [26]. Другими ключевыми критериями включения были возраст от 18 до 75 лет, удовлетворительное общее состояние: 0–2 по шкале ECOG (Eastern Cooperative Oncology Group), адекватная функция органов и ожидаемая продолжительность жизни более 12 недель. В ключевые критерии невключения входили признаки декомпенсированного стеноза желудка, кишечной непроходимости, активного желудочно-кишечного кровотечения, перфорации, требовавшие хирургического или иного вмешательства, а также выявленное метастатическое поражение центральной нервной системы, наличие неконтролируемых сопутствующих заболеваний, в т.ч. психических, иные социальные и физические причины, которые, по мнению исследователей, не позволяли пациенту соблюдать условия протокола. Все пациенты перед включением в протокол подписывали добровольное информированное согласие. Полные критерии приемлемости представлены в протоколе исследования.
Пациенты были рандомизированы случайным образом в соотношении 1:1 в группу FOLFIRINOX (иринотекан в дозе 180 мг/м2 в 1-й день, оксалиплатин в дозе 85 мг/м2 в 1-й день, лейковорин – 200 мг/м2 в 1-й день, 5-ФУ – 250 мг/м2 струйно в 1-й день с последующей 46–48-часовой инфузией 5-ФУ в дозе 2200 мг/м2) или mFOLFOX6 (оксалиплатин 85 мг/м2 в 1-й день, лейковорин – 400 мг/м2 в 1-й день, 5-ФУ – 400 мг/м2 струйно в 1-й день с последующей 46–48-часовой инфузией 5-ФУ в дозе 2400 мг/м2). Повторяемость – каждые 2 недели. ХТ сопровождалась стандартной антиэметической профилактикой, включившей блокаторы 5-HT3 рецепторов и дексаметазон, при недостаточной эффективности добавляли блокатор NK1-рецепторов апрепитант и оланзапин. Факторами стратификации являлись возраст (менее 65 или ≥65 лет), степень дифференцировки G1+2 против G3+перстневидноклеточный), распространенность процесса (1–2 зоны отдаленного метастазирования против ≥3), наличие или отсутствие первичной опухоли желудка, локализация первичной опухоли (кардиоэзофагеальный переход или другие отделы желудка), функциональный статус по шкале ECOG 0–1 против 2.
В отсутствие признаков прогрессирования или неприемлемой токсичности планировалось проведение 9 двунедельных циклов ХТ с последующим наблюдением до прогрессирования заболевания. Лечение прекращали досрочно при регистрации:
1) прогрессирования процесса,
2) неприемлемой токсичности или декомпенсации сопутствовавших заболеваний, 3) при интервале между курсами более 4 недель, 4) отказе пациента, 5) согласно мнению лечащего врача. Редукция доз препаратов проводилась согласно протоколу клинического исследования. Токсичность лечения оценивали на каждом цикле лечения согласно CTC AE version 5.0 [27], эффективность – каждые 3 цикла по критериям RECIST 1.1 [26].
Основная цель исследования – продемонстрировать преимущество режима mFOLFIRINOX по сравнению с режимом mFOLFOX6 в отношении ВБП. С целью уменьшения риска прогрессирования до значения отношения рисков (ОР) 0,73 и увеличения мВБП с 5,0 до 6,74 месяца при мощности исследования 82%, α=0,05 (односторонний р: FOLFIRINOX>mFOLFOX), потерь данных 10% пациентов необходимо включить в исследование по 163 больных в каждую группу. Длительность набора – 4 года, длительность исследования – 5 лет. Вторичными конечными точками исследования являлись мОВ, частота объективного ответа (ЧОО), токсичность и переноси-мость.
Описательная статистика использовалась для оценки результатов в терминах средних значений, частот и процентов, процентные данные по НЯ считали из расчета степени токсичности 0+1+2+3+4=100%. Для сравнения данных выборок, в частности данных по НЯ, использовали χ2-критерий. Значимость различий в средних показателях между выборками оценивали с помощью t-критерия Стьюдента, различие считали значимым при p<0,05.
Статистический анализ полученных результатов выполнен с помощью статистической программы IBM SPSS Statistics v. 23.0.
Результаты
С ноября 2019 по декабрь 2022 г. в исследование включены 210 пациентов, в анализ токсичности включены 138 больных, завершивших лечение: 58 больных в лечебной группе FOLFIRINOX и 80 – в группе лечения mFOLFOX6. Два пациента в группе FOLFIRINOX и 1 в группе mFOLFOX6 не получали лечения и исключены из анализа. Пациенты обеих групп хорошо сбалансированы по основным прогностическим факторам.
Подробная характеристика больных приведена в |табл. 1.
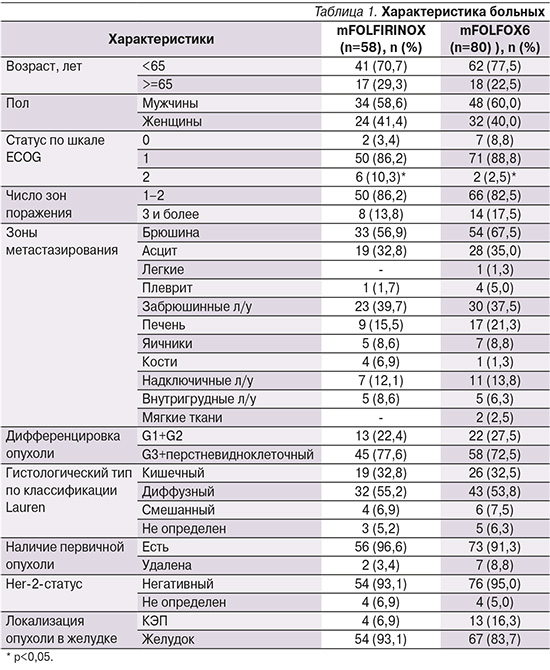
Отметим, что среди включенных пациентов отсутствовали пациенты с подтвержденным Her2-позитивным статусом. В группе пациентов, получавших FOLFIRINOX (n=58), негативный Her2-статус отмечен у 54 (93,1%) пациентов, не определен у 4 (6,9%), в группе пациентов, получавших mFOLFOX6 (n=80), отрицательный Her2-статус определен у 76 (95,0%).
Токсичность режимов ХТ первой линии представлена в табл. 2.
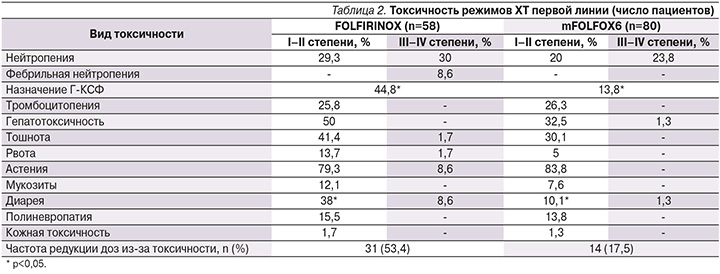
Наиболее частым видом токсичности была гематологическая. В группе пациентов, получавших FOLFIRINOX, нейтропения I–II степеней зарегистрирована у 29,3% больных, нейтропения III–IV степеней у 30%, фебрильная нейтропения у 8,6% пациентов. В группе пациентов, получавших режим mFOLFOX6, нейтропения I–II степеней зарегистрирована у 20%, III–IV степеней у 23,8%, фебрильная нейтропения не наблюдалась. Первичная профилактика нейтропении гранулоцитарными колониестимулирующими факторами (Г-КСФ) с первого курса лечения не предусматривалась. Их назначение в процессе лечения триплетом потребовалось 44,8% больных, что существенно чаще, чем при использовании mFOLFOX6 (13,8%). Различия в частоте использования Г-КСФ между двумя выборками были статистически значимыми (p<0,05). Инфекционных осложнений или летальных исходов не отмечено.
В целом профиль токсичности режимов FOLFIRINOX и mFOLFOX6 в первой линии дРЖ/КЭП совпадает.
В группе, получавшей триплет, тромбоцитопения I–II степеней зарегистрирована в 25,8% случаев, III–IV степеней не отмечена. Среди больных, получавших двукомпонентный режим, тромбоцитопения I–II степеней зарегистрирована у 26,3% пациентов, III–IV степеней не отмечена.
Основными негематологическими НЯ при ХТ в режиме FOLFIRINOX были гепатотоксичность I–II степеней у 50% пациентов, III–IV степеней не наблюдали, тошнота I–II степеней у 41,4%, III–IV степеней у 1,7%, рвота I–II степеней у 13,7%, III–IV степеней у 1,7%, астения I–II степеней зарегистрирована в 79,3% случаев, III–IV степеней в 8,6%, мукозиты I–II степеней в 12,1%, III–IV степеней не отмечены, диарея I–II степеней отмечена у 38% больных, III степени у 8,6%, полиневропатия I–II степеней у 15,5%, III–IV степеней не отмечена, кожная токсичность I–II степеней выявлена у 1,7% больных, III–IV степеней не зарегистрирована. На фоне ХТ в режиме mFOLFOX6 отмечены следующие негематологические НЯ: гепатотоксичность I–II степеней у 32,5% пациентов, III–IV степеней у 1,3%; диарея I–II степеней у 10,1%, III–IV степеней у 1,3%; тошнота I–II степеней у 30,1%, рвота I–II степеней у 5%, астения I–II степеней у 83,8%, мукозиты I–II степеней у 7,6%, полиневропатия I–II степеней у 13,8%, кожная токсичность I–II степеней зарегистрирована у 1,3% больных, III–IV степеней вышеуказанных НЯ в этой группе пациентов не наблюдали. Обращает на себя внимание превалирование гепатотоксичности в группе, получавшей FOLFIRINOX, по сравнению с mFOLFOX6 (50 против 33,8%), что было статистически незначимо. Также существенно чаще при использовании триплета, чем при использовании дуплета, отмечена диарея: 46,6 против 11,4%, частота диареи I–II степеней была статистически значимо выше у пациентов, получавших режим FOLFIRINOX, чем при режиме mFOLFOX6 (38 и 10,1%; p<0,05).
В остальном различия по НЯ у пациентов, получавших FOLFIRINOX или mFOLFOX6, были сходными и статистически значимых различий не найдено (табл. 2).
Частота редукций доз из-за токсичности была существенно выше при использовании режима FOLFIRINOX. Редукция доз препаратов по любой причине выполнена 53,4% больных, получавших режим FOLFIRINOX, и 17,5% пациентов, получавших режим mFOLFOX6. Среди пациентов, получавших лечение в режиме FOLFIRINOX, все запланированные курсы проведены 90,2% больных; в группе пациентов, получавших mFOLFOX6, 84,9%. Несмотря на высокую частоту редукций доз, НЯ были скорректированы и случаев прекращения лечения из-за токсичности мы не отметили. Причины прекращения лечения представлены в табл. 3.

Основными причинами прекращения ХТ в режиме FOLFIRINOX были прогрессирование болезни (19% пациентов), кровотечение (3,4%), в режиме mFOLFOX6 – прогрессирование болезни (32,5% пациентов), желудочное кровотечение (3,8%), перфорация желудка (1,3% больных). Следует отметить, что прогрессирование болезни служило причиной прекращения лечения чаще при лечении в режиме mFOLFOX6, чем FOLFIRINOX (32,5 против 19,0%), различия были статистически не значимы.
Обсуждение
Токсичность режима FOLFIRINOX в первой линии лечения пациентов с дРЖ/КЭП была приемлемой. Частота назначения Г-КСФ при применении FOLFIRINOX оказалась несколько выше по сравнению с аналогичными данными H. Park et al.: 44,8 против 34% [14]. По сравнению с исследованием Н. Park et al. [14] показатели токсичности в нашем исследовании были несколько ниже: нейтропения имела место у 59,3% в нашем исследовании и у 91% пациентов в работе Н. Park et al., в т.ч. III–IV степеней соответственно у 30 и 79%, диарея всех степеней составила 46,6% в нашем исследовании и 63%, по данным H. Park et al., в т.ч. диарея III степени составила 8,6 и 13,4% соответственно, полинейропатия отмечена у 15,5 и 61% пациентов соответственно, модификация доз вводимых препаратов при лечении в режиме FOLFIRINOX или отсрочка очередного курса лечения у 53,4 и 84% соответственно.
По сравнению с режимом mFOLFOX6 в нашем исследовании частота НЯ при ХТ в режиме FOLFIRINOX в целом была выше, однако статистически значимое увеличение получено лишь для диареи I–II степеней тяжести (38 против 8,6%), частота применения Г-КСФ при режиме FOLFIRINOX более чем в 3 раза была выше, чем при режиме mFOLFOX6 (44,8 и 13,8%; p<0,05). Выраженные НЯ III–IV степеней тяжести также чаще отмечены при режиме FOLFIRINOX, чем при режиме mFOLFOX6, однако все различия были статистически незначимы. Все НЯ были контролируемыми, и случаев прекращения ХТ вследствие развития НЯ в настоящем исследовании зарегистрировано не было.
Несмотря на более высокую частоту НЯ, первую линию ХТ в режиме FOLFIRINOX в полном объеме удалось провести не меньшему числу больных, чем в режиме mFOLFOX6: 90,2 и 84,9% пациентов соответственно. Своевременная модификация доз в сочетании с адекватной симптоматической и сопроводительной терапией, вторичной профилактикой нейтропении Г-КСФ обеспечила проведение всех запланированных курсов ХТ практически всем больным. Следует отметить также, что частота прекращения лечения вследствие прогрессирования болезни была почти в 2 раза выше при режиме mFOLFOX6, чем при FOLFIRINOX: 32,5 и 19%.
Заключение
Промежуточный анализ переносимости и токсичности свидетельствует о приемлемой токсичности режима FOLFIRINOX, возможности проведения полноценной первой линии ХТ 90% больных дРЖ/КЭП без жизнеугрожающих осложнений лечения. Набор пациентов в исследование продолжается.
Вклад авторов. Н.С. Бесова, И.С. Стилиди – концепция и дизайн исследования. Е.С. Обаревич, Д.А. Гаврилова – сбор и обработка материала. Е.С. Оба-ревич – статистическая обработка данных. Г.Г. Макиев, Д.А. Гаврилова – написание текста. Н.С. Бесова, А.А. Трякин – редактирование.



