Введение
Последние 10–15 лет диабетологической науки и практики отмечены бурным ростом числа сахароснижающих препаратов (ССП) и их качественным обновлением, т.е. появлением лекарств с принципиально новыми механизмами действия: агонистов рецепторов глюкагоноподобного пептида-1 (арГПП-1), ингибиторов дипептидилпептидазы-4 (ДПП-4) и ингибиторов натрий-глюкозного транспортера 2-го типа (НГЛТ2). Прочно укрепилась концепция оценки отдаленной безо-
пасности и эффективности ССП, согласно которой они должны доказать не только традиционное влияние на уровень глюкозы в крови, но и свое как минимум нейтральное (безопасность), а лучше – положительное (эффективность) действие на клинические конечные сердечно-сосудистые точки. Таково в настоящее время требование регуляторных органов к регистрации новых лекарств для лечения сахарного диабета 2 типа (СД2) [1, 2].
Это требование обусловлено следующими соображениями:
- именно сердечно-сосудистые заболевания (ССЗ) служат ведущей причиной смерти больных СД [3];
- улучшение показателей гликемии при СД2 практически не влияет на кардиологический прогноз и продолжительность жизни [4, 5];
- ССП не могут «по умолчанию» считаться безопасными для сердечно-сосудистой системы (ССС), о чем свидетельствует история толбутамида, фенформина, росиглитазона, мураглитазара и др. [6], т.е. их кардиобезопасность надо доказывать.
Данный обзор посвящен лираглутиду и его разнообразным эффектам, обусловливающим повышенный интерес к нему не только эндокринологов, но и кардиологов, и терапевтов. Здесь мы постараемся проанализировать последнюю информацию по кардиоэффективности и кардиобезопасности лираглутида по сравнению с другими ССП.
Лираглутид как сахароснижающий препарат
Лираглутид (Виктоза®; Ново Нордиск, Дания) – инъекционный арГПП-1, аналог ГПП-1 человека, который связывается с альбумином в межклеточной жидкости после подкожного введения и благодаря этому оказывает сахароснижающее действие длительностью около суток. Препарат давно и хорошо известен российским эндокринологам; механизмы действия ГПП-1 и арГПП-1 на углеводный обмен уже рассматривались в русскоязычных публикациях [7].
Программа клинических исследований LEAD показала, что лираглутид снижал уровень гликозилированного гемоглобина (HbA1c) на 1,6%, при этом до 66% больных достигали целевого уровня HbA1c<7% [8]. В наиболее крупном и продолжительном исследовании LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) на фоне среднего периода наблюдения в 3,8 года и сопутствующей стандартной сахароснижающей терапии риск ухудшения контроля гликемии, связанный с неуклонным прогрессированием заболевания, в группе плацебо был значительно больше (n=3988, 85,4%), чем в группе лираглутида (n=3202, 68,6%); при этом отношение риска (ОР) для декомпенсации СД при лечении лираглутидом оказалось практически вдвое ниже, чем на плацебо (ОР=0,50; 95% доверительный интервал [ДИ] – 0,48–0,53; p< 0,001). Среднее время удержания компенсации СД в группе лираглутида было в 3,57 раза больше, чем в группе плацебо, причем в группе плацебо 50% пациентов оказались декомпенсированными уже через 4,8 месяца, тогда как в группе лираглутида ухудшение углеводного обмена отмечено через 19,5 месяца [9]. Сахароснижающая эффективность лираглутида сохраняется на протяжении более 2 лет [10].
Прямые сравнительные рандомизированные исследования выявили, что по сахароснижающему эффекту лираглутид не уступает большинству препаратов сравнения или превосходит их (табл. 1). Интерпретируя статистически значимые различия между препаратами сравнения, нужно помнить, что минимальной клинически значимой (т.е. влияющей на развитие микроангиопатий) разницей HbA1c является ≥0,5% [11, 12].
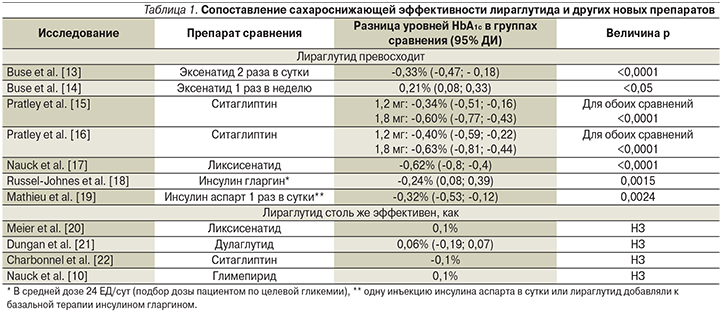
Еще в одном сравнительном исследовании лираглутид в среднесуточной дозе 1,74 мг уступил инсулину гларгин в среднесуточной дозе 54 ЕД по среднему снижению HbA1c (-1,94% на гларгине и -1,79% на лираглутиде; p = 0,019) (оба препарата добавляли к терапии метформином±производные сульфонилмочевины). Однако по числу больных, достигших целевых значений HbA1c<7%, оба препарата были одинаковыми (48,4 против 45,9%). Кроме того, терапия гларгином приводила к прибавке массы тела +2,0 (4,0) кг, а терапия лираглутидом сопровождалась потерей массы тела -3,0 (3,6) кг для лираглутида (p<0,001) [23]. В непрямом мета-анализе 16 исследований [24] показано, что обе дозы лираглутида значимо превосходили различные ингибиторы НГТЛ2 по динамике уровня HbA1c и доле пациентов, достигших HbA1c<7%.
Таким образом, по совокупности имеющихся данных можно считать, что лираглутид сейчас является одним из наиболее эффективных неинсулиновых ССП. Высокая эффективность в плане коррекции гипергликемии сопровождается низкой частотой гипогликемий, что было показано во всех процитированных выше исследованиях. Другим преимуществом лираглутида в отношении углеводного обмена является его положительное влияние на β-клетки. Хорошо известно, что СД2 характеризуется прогрессирующей потерей массы и нарушением функции этих клеток. Несколько исследований показали, что лираглутид не только оказывает на них защитное действие, уменьшает их апоптоз, сохраняет и увеличивает массу β-клеток, но и улучшает инсулинсекреторную функцию, а также снижает повышенную секрецию глюкагона, тем самым способствуя не только лучшему контролю гликемии, но и увеличению чувствительности к инсулину [25–27]. Несомненно, что сахароснижающие свойства лираглутида обусловлены и его влиянием на массу тела и пищевое поведение (см. далее).
Плейотропные эффекты лираглутида на факторы риска атеросклероза
Поскольку рецепторы ГПП-1 расположены не только в поджелудочной железе, доклинические и клинические исследования арГПП-1 выявили множество других свойств, не ограничивающихся углеводным обменом.
Масса тела. Наиболее очевидным несомненно можно считать действие на важнейший фактор риска атеросклероза – ожирение. Так, в исследовании «SCALE диабет» при лечении больных СД2 в течение 56 недель лираглутидом в дозе 3 мг/сут масса тела снижалась на 6,1% от исходной, в дозе 1,8 мг – на 4,7%, в то время как на плацебо снижение массы тела составило 1,9% [28]. В прямых сравнительных исследованиях с другими препаратами инкретинового ряда лираглутид снижал массу тела значимо больше, чем ситаглиптин [16], дулаглутид [21], эксенатид продленного действия [14] и не уступал по этому параметру ликсисенатиду [17] и эксенатиду короткого действия [13].
Механизмы влияния лираглутида на массу тела многообразны. Все арГПП-1 снижают массу тела в диапазоне от 1,5 до 6,0 кг за 30 недель терапии. В настоящее время получены убедительные данные, подтверждающие, что ГПП-1 является сильным анорексигенным гормоном, физиологическим регулятором аппетита, усиливающим чувство сытости и снижающим чувство голода. При этом арГПП-1 с малой молекулярной массой, такие как лираглутид, эксенатид и ликсисенатид, проникая через гематоэнцефалический барьер, обладают доказанно бóльшим влиянием на центры насыщения и голода в гипоталамусе, чем аГПП-1 с большой молекулярной массой, и сильнее снижают массу тела [29]. Действие лираглутида на энергетический баланс организма – преимущественно центральное [30]. Рецепторы к ГПП-1 имеются в многочисленных отделах головного мозга, включая кору, гипоталамус, таламус, подкорковые ядра и ряд других зон [31–32]. Он влияет на дугобразные ядра гипоталамуса, где находятся анорексигенные нейроны, продуцирующие проопиомеланокортин (ПОМК) и кокаин-амфетамин-регулируемый транскрипт (КАРТ) [33]. Воздействуя на гипоталамус, лираглутид уменьшает аппетит, влечение к вкусной пище, в конечном счете снижая потребление калорий и дополнительно снижая гликемию. Ингибирующий эффект ГПП-1 на получение удовольствия от еды опосредуется через вентральную тегментную зону и n. accumbens, которые входят в мезолимбическую «систему вознаграждения» [34]. В эксперименте на животных лираглутид, воздействуя на гипоталамические нейроны, активирует бурую жировую ткань с усилением термогенеза и, возможно, способствует трансформации бурой жировой ткани в белую. Не менее интересным представляется и возможное уменьшение воспалительного процесса в гипоталамусе. Асептическое воспаление гипоталамуса может развиваться на фоне высокожирового питания и непосредственно влияет на нейрональные взаимодействия, контролирующие прием пищи и энергетический баланс. Устранение этого воспаления может способствовать восстановлению нормального энергетического баланса в той его части, которая регулируется гипоталамусом [35].
Эфферентные импульсы из гипоталамуса и ствола мозга регулируют функциональное состояние желудочно-кишечного тракта, тормозя его моторику и секрецию желудочной кислоты; что также влияет на пищевое поведение и углеводный обмен [34]. Кроме того, замедление моторики желудка развивается и при прямой стимуляции рецепторов ГПП-1 в желудке – эффект, очень выраженный у лираглутида [36]. В свою очередь снижение скорости опорожнения желудка может сопровождаться его некоторым растяжением со стимуляцией механорецепторов желудочной стенки и последующей активацией ганглиев блуждающего нерва; последние «сигнализируют» в нейроны ядра одиночного тракта (n. tractus solitarii) о наступлении насыщения («чувство наполненности желудка») [34].
Таким образом, конечный результат – изменение пищевого поведения и снижение массы тела – при лечении лираглутидом опосредован целым комплексом центральных и периферических регуляторных механизмов, которые запускаются стимуляцией рецепторов ГПП-1.
Артериальное давление (АД) и другие компоненты метаболического синдрома.
Лираглутид, как и другие арГПП-1, снижает АД у больных СД [37–38].
В мета-анализе B. Wang et al. [39] лираглутид в дозе 1,2 мг/сут по сравнению с плацебо снижал систолическое АД на 5,6 мм рт.ст. (95% ДИ – -5,84 – -5,36; p<0,00001), в дозе 1,8 мг/сут – на 4,49 мм рт.ст. (95% ДИ – -4,73 – -4,26; p<0,00001). Однако считается, что снижение АД опосредовано не столько уменьшением массы тела, сколько независимым действием лираглутида на систему предсердного натрийуретического пептида (ANP). Предполагают, что, активируя рецепторы ГПП-1 в кардиомиоцитах предсердия, лираглутид влияет на концентрации ANP и связанные с ним релаксацию гладкой мускулатуры сосудистой стенки (вазодилатация), а также выведение натрия с мочой с уменьшением объема внеклеточной жидкости [40–41]. Интересно, что помимо регуляции водно-солевого баланса ANP усиливает липолиз, способствует термогенезу в адипоцитах, усиливает окисление жиров и окислительное фосфорилирование в миоцитах, а также стимулирует глюкозозависимую секрецию инсулина [42–45]. На этом примере хорошо видно, как влияние лираглутида на один из биологически активных регуляторов ССС – ANP – пересекается с механизмами влияния препарата на жировой и углеводный обмены.
Как и все арГПП-1, лираглутид увеличивает частоту сердечных сокращений в среднем примерно на 2 уд/мин [46–47], но, как можно судить по представленным дальше результатам клинических исследований, это не препятствует его кардиопротективному эффекту.
Уменьшая массу тела, лираглутид закономерно снижает показатели окружности талии, уровни общего холестерина и холестерина липопротеидов низкой плотности, триглицеридов и повышает уровни холестерина липопротеидов высокой плотности [18, 25]. Вместе с улучшением контроля гликемии это ведет к уменьшению числа пациентов с метаболическим синдромом на 26% за 18 месяцев терапии [48]. Однако лираглутид обладает и не зависимым от снижения массы тела гиполипидемическим эффектом; возможно, это происходит благодаря влиянию ГПП-1 на энтероциты, отвечающие за всасывание липидов и сборку хиломикронов, а также через центральные механизмы [49].
Системный воспалительный ответ и эндотелиальная дисфункция. Системное воспаление и тесно связанная с ним эндотелиальная дисфункция – важные патофизиологические звенья атеросклероза, СД2 и ожирения. Их выраженность может уменьшаться не только за счет снижения массы тела и улучшения гликемии, но и напрямую через влияние лираглутида на рецепторы ГПП-1 в клетках сосудов, крови, иммунной системы и других органов [50]. Под влиянием арГПП-1 усиливается эндотелийзависимая вазодилатация, что указывает на активацию эндотелиальной системы синтеза оксида азота [51]. Кроме того, в эндотелии сосудов стимуляция рецепторов ГПП-1 уменьшает количество рецепторов к конечным продуктам гликирования, потенциально ослабляя влияние хронической гипергликемии на белки сосудистой стенки [52]. Там же ГПП-1 оказывает противовоспалительное действие благодаря уменьшению концентрации свободных кислородных радикалов [53–54]. В эксперименте лираглутид напрямую снижает продукцию провоспалительных цитокинов: фактора некроза опухоли α, интерлейкина-1b (ИЛ-1b) и ИЛ-6 макрофагами, PAI-1, внутриклеточной молекулы адгезии-1 и VCAM-1 в эндотелии сосудов человека [50]. Снижение маркеров воспаления и атеротромбогенеза (высокочувствительного С-реактивного белка, фибриногена, sVCAM-1, PAI-I, свободных жирных кислот) было выявлено и в клинических исследованиях на фоне лечения лираглутидом [18, 55]. Терапия лираглутидом в течение 18 месяцев сопровождалась уменьшением толщины комплекса интима-медиа в сонной артерии – известного суррогатного параметра атеросклероза [48].
Кардиопротективное действие лираглутида по клиническим конечным точкам
Плейотропное действие лираглутида на углеводный и липидный обмены, массу тела и окружность талии, АД, показатели системного воспалительного ответа и эндотелиальной дисфункции лежит в основе суммарного положительного действия препарата на сердечно-сосудистую систему.
Основополагающим в отношении действия лираглутида на клинические конечные точки стало двойное слепое рандомизированное плацебо-контролируемое исследование LEADER, или Эффект и действие лираглутида при диабете: результаты оценки сердечно-сосудистых исходов [56]. В него вошли более 9300 больных СД2, 81% из которых имели диагноз ССЗ атеросклеротического генеза или возраст ≥60 лет в сочетании минимум с одним сердечно-сосудистым фактором риска. Главная составная конечная точка включала сердечно-сосудистую смертность, несмертельный инфаркт и несмертельный инсульт. Исследование подтвердило безопасность препарата для ССС и более того, его сердечно-сосудистую эффективность и возможность увеличения продолжительности жизни. Частота главной конечной точки на фоне лечения лираглутидом снизилась на 13% (р=0,01). Число больных, которых необходимо лечить лираглутидом в течение 3,8 года для предотвращения одного клинического события, относящегося к главной конечной точке (NNT, number needed to treat), равнялось 55. Это снижение было обусловлено снижением относительного риска сердечно-сосудистой смерти на 22% (NNT=79). Что самое важное, в исследовании снизился риск общей смертности на 15%, т.е. для сохранения одной жизни необходима терапия лираглутидом 71 больного на протяжении более 3 лет [56]. Напомним, что значения NNT менее 100 принято считать критерием высокой эффективности конкретного вида лечения по клиническим конечным точкам.
Целесообразно подробнее проанализировать, какие именно пациенты лучше всего реагировали на терапию лираглутидом по сердечно-сосудистым конечным точкам. Из 9340 больных СД2, включенных в исследование, у 3692 (39,5%) в анамнезе имелся инфаркт миокарда или инсульт, еще 3083 (33,0%) имели подтвержденные диагнозы ССЗ атеросклеротического генеза, но ранее не переносили инфаркта или инсульта, и у 2565 (27,5%) пациентов были только факторы риска атеросклероза. Лираглутид статистически значимо уменьшал частоту главных неблагоприятных сердечно-сосудистых событий у больных, уже перенесших инфаркт миокарда или инсульт: 322 (17,3%) из 1865 на лираглутиде против 372 (20,4%) из 1827 на плацебо (ОР=0,85, 95% ДИ – 0,73–0,99) и у пациентов с уже имевшимися ССЗ, но без инфаркта или инсульта в анамнезе: 158 (10,3%) из 1538 в группе лираглутида против 199 (12,9%) из 1545 больных в группе плацебо (ОР=0,76, 95% ДИ – 0,62–0,94). В отличие от этого у больных, имевших только факторы риска, терапия лираглутидом не сопровождалась значимым изменением главной конечной точки (ОР=1,08, 95% ДИ – 0,84–1,38). Аналогичная неодинаковая динамика была выявлена и в отношении вторичных конечных точек [57]. Таким образом, кардиоэффективность лираглутида реализуется у больных СД2, уже имеющих диагноз атеросклеротических ССЗ, вне зависимости от того, был ли у них инфаркт или инсульт, или нет.
Известно, что атеросклероз более чем одного сосудистого бассейна является мощным независимым предиктором сердечно-сосудистых событий [58]. Поэтому возникает еще один интересный в кардиологическом отношении вопрос: различается ли кардиоэффективность лираглутида у пациентов с разной степенью вовлеченности сосудистых бассейнов в атеросклероз? Для ответа на него сравнили результаты исследования LEADER в трех группах больных: без клинических проявлений атеросклероза, с атеросклеротическим поражением одного сосудистого региона (коронарного, или церебрального, или периферического) и с полирегионарным сосудистым поражением, т.е. атеросклерозом в двух и более бассейнах [59]. К началу исследования подтвержденные ССЗ имелись у 6775 (72,5%) больных; у 23% из них был полирегионарный атеросклероз, у 77% – монорегионарный. У пациентов с полирегионарным поражением риск развития сердечно-сосудистых событий был значимо выше, чем при поражении одного сосудистого бассейна: ОР для главной составной сердечно-сосудистой точки составил 1,52 (95% ДИ – 1,33–1,73), для расширенной главной точки – 1,45 (95% ДИ – 1,31–1,62), сердечно-сосудистой смерти – 1,41 (95% ДИ – 1,13–1,75). Влияние лираглутида на состояние ССС в зависимости от распространенности атеросклероза представлено в табл. 2.
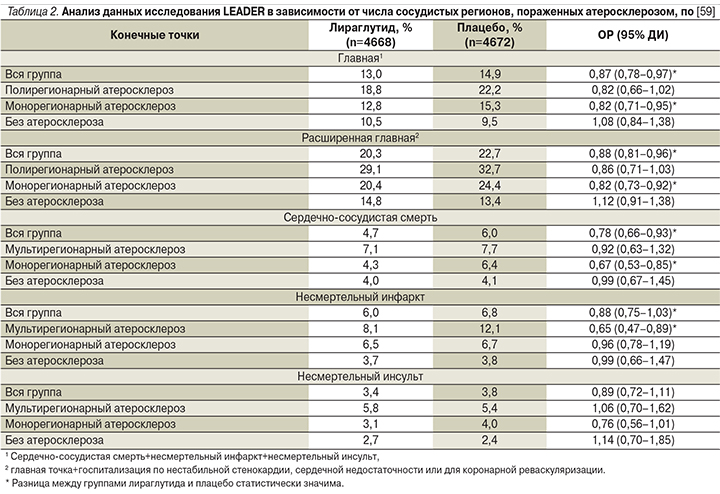
Как видно из представленных данных, терапия лираглутидом значимо снижала риск главной составной сердечно-сосудистой конечной точки при поражении одного бассейна (ОР=0,82, 95% ДИ – 0,71–0,95) и чуть не дотягивала до значимого снижения у лиц с полирегионарным поражением (это обусловлено большим доверительным интервалом, т.е. разбросом данных). Абсолютное же снижение риска при полирегионарном поражении было даже больше, чем при монорегионарном. Для расширенной главной конечной точки и для сердечно-сосудистой смертности были выявлены аналогичные закономерности. Лираглутид снижал частоту несмертельного инфаркта именно при полирегионарном атеросклерозе, а вот по частоте несмертельного инсульта значимой динамики не было ни в одной из подгрупп, хотя при монорегионарном атеросклерозе снижение риска инсульта лишь немного не дотянуло до статистической значимости.
В отличие от эффективности терапии лираглутидом больных атеросклерозом у пациентов без развившегося атеросклеротического поражения ССС лираглутид не снижал риска сердечно-сосудистых событий. Это может быть связано как с истинным отсутствием эффекта у этих больных, так и (скорее) с тем, что в этой подгруппе сердечно-сосудистый риск изначально был существенно ниже и для его изменения необходимо было более длительное наблюдение или больший размер выборки [59].
В ходе отдельного, не предусмотренного протоколом анализа результатов исследования LEADER у больных пожилого и старческого возраста показано снижение риска сердечно-сосудистых событий и общей смертности в этой наиболее уязвимой подгруппе с наиболее высоким сердечно-сосудистым риском, причем положительный эффект у пациентов старше 75 лет был более выраженным, чем у пациентов 60–74 лет [60].
Поскольку ранее поднимались вопросы о кардиобезопасности ингибиторов ДПП-4, также принадлежащих к классу инкретинов [61], важно, что в исследовании LEADER лираглутид не только не увеличил число госпитализаций в связи с сердечной недостаточностью (СН), но даже количественно его уменьшил, хотя разница не достигла статистической значимости [56].
В другом клиническом рандомизированном плацебо-контролируемом исследовании 2-й фазы изучали влияние лираглутида на показатели клинической стабильности у больных СН с низкой фракцией выброса (средняя – 25%), недавно перенесших госпитализацию в связи с острой СН; 59% пациентов страдали СД2 [62]. Главной конечной точкой в исследовании был суммарный балл стабильности, который рассчитывался с учетом времени до летального исхода, времени до следующей госпитализации по поводу СН и усредненного по времени процентному изменению уровня N-терминального про-B-натрийуретического пептида от исходной точки до 180-го дня. Было установлено, что у больных СН с низкой фракцией выброса лираглутид не влиял на клиническое течение и не изменял параметры смертности и госпитализации по СН. Эти результаты были одинаковыми у больных как СД, так и без него. В другом небольшом исследовании больных СН как с СД, так и без него лираглутид не влиял на фракцию выброса, хотя несколько увеличивал частоту аритмий, включая суправентрикулярную тахикардию, что объясняется локализацией рецепторов ГПП-1 в синоатриальном узле [63].
Еще один важный аспект изучения лираглутида – оценка его потенциального действия в отношении диабетических язвенных дефектов стопы и их осложнений. Эта необходимость диктуется тем, что в ходе клинического исследования одного из новых препаратов группы ингибиторов НГЛТ2 – канаглифлозина – показано значимое увеличение частоты ампутаций нижних конечностей [64]. Анализируя результаты исследования LEADER, K. Dhatariya et al. показали, что за 3,8 года лечения хотя бы один эпизод диабетического язвенного дефекта стопы в группах лираглутида и плацебо отмечался с одинаковой частотой: 3,8 против 4,1% соответственно (ОР=0,92, 95% ДИ – 0,75–1,13; р=0,41); не было и различий в частоте инфекций стопы (в т.ч. глубоких) и периферической реваскуляризации. Вместе с тем в группе лираглутида клинически и статистически значимо уменьшилось число ампутаций – на 35% по сравнению с плацебо (ОР=0,65, 95% ДИ – 0,45–0,95; р=0,03]). Это крайне интересная находка, которая, по мнению авторов работы, нуждается в дальнейшем подтверждении [65].
Сравнительный анализ кардиобезопасности и кардиоэффективности новых классов ССП
Время, когда для сравнительной оценки ССП опирались только на суррогатные биохимические и физиологические («мягкие») критерии – показатели углеводного и липидного обменов, массу тела, пожалуй, прошло. Сегодня при выборе из всего ассортимента лекарственных средств все бóльшую роль играют «твердые» клинические конечные точки – смертность, осложнения, качество жизни, по которым судят о клинической пользе каждого препарата, складывающейся из соотношения его эффективности и безопасности. Сравнительный анализ новых ССП именно с этих позиций представлен в табл. 2 (здесь мы проанализируем лишь данные, касающиеся кардиологических конечных точек; почечные исходы не являются предметом данной публикации).
Как видно из данных табл. 3, у больных СД2 четыре основных ингибитора ДПП-4 показали в принципе сходные результаты: они не снижали и не увеличивали частоту главной составной сердечно-сосудистой конечной точки и не влияли отдельно ни на сердечно-сосудистую смертность, ни на несмертельные инфаркты и инсульты. Таким образом, можно считать, что все эти препараты обладают сердечно-сосудистой безопасностью, но не улучшают кардиологический прогноз больных СД2 и тем более не влияют на общую смертность. Более того, саксаглиптин повышает риск госпитализаций в связи с СН на 0,7 абсолютного процента (или на 27 относительных процентов), что стало основанием отказаться от его применения больными ХСН III–IV функциональных классов. В таблице отсутствует вилдаглиптин, т.к. специальное исследование с клиническими сердечно-сосудистыми конечными точками по нему не проводилось – иными словами, его сердечно-сосудистая безопасность не доказана. Не было такого исследования и по госоглиптину – новому ингибитору ДПП-4, в 2018 г. зарегистрированному в России.
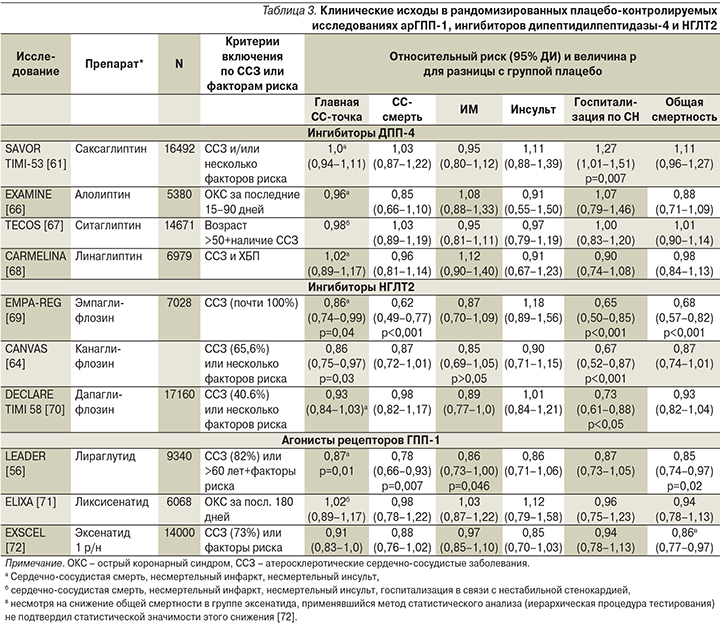
Изучение влияния ингибиторов НГЛТ2 и арГПП-1 на сердечно-сосудистые исходы при СД2 показало не только их кардиобезопасность, но и во многих случаях кардиоэффективность. Эмпаглифлозин и канаглифлозин снижали главную составную сердечно-сосудистую конечную точку и риск госпитализаций по поводу СН, дапаглифлозин – риск госпитализаций по СН, а также вторую главную сердечно-сосудистую точку (не указана в табл. 3) – комбинацию сердечно-сосудистой смерти и госпитализации по СН (ОР=0,83, 95% ДИ – 0,73–0,95; р=0,005), но только за счет уменьшения числа госпитализаций по СН [70]. Только канаглифлозин повышал риск ампутаций нижних конечностей (ОР=1,97, 95% ДИ – 1,41–2,75; р<0,001) [64]. Общую смертность снижал лишь один препарат этой группы – эмпаглифлозин. Таким образом, результаты оценки сердечно-сосудистой безопасности и особенно эффективности ингибиторов НГЛТ2 выглядят более неоднородными, чем ингибиторов ДПП-4.
Что касается арГПП-1, то ликсисенатид и эксенатид пролонгированного действия оказались безопасными для больных СД2 с атеросклеротическими ССЗ, включая острый коронарный синдром (ОКС), но не были эффективными в плане улучшения сердечно-сосудистого прогноза или прогноза жизни (табл. 3) [71–72]. В отличие от них лираглутид, как уже говорилось в предыдущем разделе статьи, продемонстрировал сердечно-сосудистую эффективность, уменьшая сердечно-сосудистую смертность, и, что еще более важно, общую смертность, т.е. реально помогая пациентам с СД2 и атеросклеротическими ССЗ прожить дольше (табл. 3) [56].
Еще не опубликованы, но уже анонсированы результаты исследования дулаглутида (REWIND), который у 9901 больного СД2 (лишь 30% имели ССЗ) длительностью более 5 лет снижал частоту составной конечной сердечно-сосудистой точки (сердечно-сосудистая смерть, несмертельный инфаркт и несмертельный инсульт), не влияя на общую смертность [73]. Следовательно, и этот класс ССП продемонстрировал существенную гетерогенность влияния на сердечно-сосудистые конечные точки [74].
В связи с этим постоянно поднимается и обсуждается вопрос: являются ли кардиобезопасность и кардиоэффективность трех новых групп ССП класс-эффектами, или каждый из препаратов нужно рассматривать по отдельности? Если говорить о кардиобезопасности, то она, несомненно, доказана для всех препаратов групп ингибиторов ДПП-4, ингибиторов НГЛТ2 и арГПП-1. Что касается кардиоэффективности, то ингибиторы ДПП-4 ею не обладают, а вот ответ на вопрос о класс-эффектах ингибиторов НГЛТ2 и арГПП-1 дать непросто. Возможно, каждый препарат внутри класса действительно обладает своими особенными свойствами, обусловленными различиями в строении молекулы, фармакокинетике и пр. Однако не исключено, что разные результаты обусловлены характеристиками пациентов, участвовавших в исследовании (разные длительность СД, исходные уровни HbA1c, процент больных ССЗ) или только факторами риска, а также разными продолжительностью исследований и размером выборок, следовательно, неодинаковой статистической мощностью [74]. Ведь статистическая мощность каждого исследования (не путать со статистической значимостью разницы между группами), а значит, и размер выборки при написании протокола исследований рассчитывались только для главной конечной точки, но не для отдельных ее компонентов и не для вторичных точек, поэтому отсутствие межгрупповой разницы по этим параметрам не может считаться надежным выводом. Кроме того, некоторые авторы задаются вопросом: не является ли положительная динамика сердечно-сосудистых конечных точек следствием действия не нового препарата, а результатом того, что в группе плацебо пациенты закономерно получали больше других ССП [75]? Ведь для оценки негликемических кардиотропных эффектов новых препаратов в исследованиях, которые приводятся в табл. 2, протоколом было предусмотрено поддержание примерно одинакового уровня HbA1c в группах сравнения. По этой причине в исследовании LEADER, например в группе плацебо, больше больных в итоге получали препараты сульфонилмочевины и инсулин [56].
Это могло послужить причиной более высокой частоты гипогликемий в группе плацебо, которая могла внести вклад в худшие сердечно-сосудистые исходы. В любом случае данный факт позволяет считать, что при необходимости улучшить контроль гликемии во многих случаях целесообразно усилить терапию лираглутидом, а не препаратами сульфонилмочевины или инсулином.
В идеале ответить на вопрос о класс-эффекте или уникальности действия каждого препарата на сердечно-сосудистые исходы могли бы прямые сравнительные рандомизированные клинические исследования разных препаратов внутри одного класса, однако в ближайшее время они вряд ли будут проведены. А пока с достаточной уверенностью можно сказать, что при выборе препарата для лечения конкретного больного СД2 следует учитывать не только влияние этого препарата на сердечно-сосудистую и общую смертность, но и то, на каком контингенте больных он изучался, т.е. каковы были критерии включения пациентов в то или иное исследование (табл. 3). Если снижения главной конечной точки удалось достичь больными, уже имевшими атеросклеротические ССЗ (вторичная профилактика), значит, данный препарат будет иметь преимущества именно в этой категории пациентов. Если же, напротив, положительные результаты получены от больных, имевших только факторы риска, но не развившихся ССЗ (первичная профилактика), то именно у таких пациентов можно ожидать аналогичного эффекта и на практике. Общей закономерностью является то, что у лиц с низким сердечно-сосудистым риском кардиоэффективность препаратов новых классов менее выражена, чем у пациентов с высоким сердечно-сосудистым риском и тем более уже развившимися ССЗ [5].
Именно по результатам исследований кардиоэффективности ССП, в частности лираглутид, получают новое показание к применению – не только контроль гликемии, но и использование для улучшения сердечно-сосудистых исходов у больных СД2. Те же соображения лежат в основе принятия решений при выборе ССП у больных с высоким сердечно-сосудистым риском. Этот подход нашел свое отображение в консенсусе Американской ассоциации диабета (ADA) и Европейской ассоциации по изучению диабета (EASD) 2018 г. [4], в котором говорится: пациентам с СД2, у которых уже имеются атеросклеротические ССЗ, в качестве сахароснижающей терапии после препарата первого ряда (метформина) рекомендуются ингибиторы НГЛТ2 или арГПП-1 с доказанными сердечно-сосудистыми преимуществами, причем на первое место среди арГПП-1 для больных, не страдающих СН, консенсус ставит лираглутид. Аналогичный алгоритм персонализации сахароснижающей терапии больных СД2 и ССЗ дан в российских клинических рекомендациях [76] и в рекомендациях Европейского кардиологического общества (ESC) [77]. Лираглутид будет иметь преимущества и для тех пациентов, у кого первостепенной проблемой является снижение массы тела и максимально возможное избегание гипогликемий [4, 76]. В тех случаях, когда перед врачом стоит вопрос, чем усилить сахароснижающую терапию больного, декомпенсированного на максимально возможных дозах пероральных ССП, предпочтение при переходе на инъекционную терапию также рекомендуется по возможности отдавать арГПП-1 и лишь потом переходить на инсулин [4].
Заключение
Появление ССП с принципиально новыми механизмами действия стало шагом вперед не только в лечении СД2 как такового, но и в решении основной проблемы этих пациентов – улучшении их кардиологического прогноза. Препараты класса арГПП-1 показали в этом отношении свою полную безопасность, а некоторые из них, в частности лираглутид, – эффективность. Важно, что лираглутид способствовал дополнительному улучшению кардиологических конечных точек и общей смертности у больных, которые уже получали стандартную терапию, направленную на коррекцию сердечно-сосудистых факторов риска – артериальной гипертензии, дислипидемии, гиперкоагуляции и т.д. Поскольку эффект лираглутида на ССС в исследовании LEADER развивался постепенно, можно предположить, что он опосредуется тормозящим влиянием препарата на развитие атеросклероза


