Введение
Рак легкого является наиболее частой причиной смерти от онкологических заболеваний у мужчин. Около 80 % случаев рака легкого составляет немелкоклеточный рак легкого (НМРЛ). До 60 % пациентов НМРЛ на момент установления диагноза имеют стадии IIIb (местнораспространенный НМРЛ) и IV (метастатический НМРЛ). Поскольку НМРЛ на данных стадиях является неизлечимым заболеванием, целью терапии у этих больных является максимальное увеличение продолжительности жизни и улучшение ее качества [1, 2, 6, 7].
Сочетание паклитаксела и карбоплатина является одной из основных схем комбинированной химиотерапии, применяемых в первой линии лечения распространенного НМРЛ [1, 3, 7, 10]. С учетом высокой стоимости этих лекарственных средств значительный интерес представляет вопрос о терапевтической эквивалентности импортных и более доступных отечественных химиопрепаратов.
В 2009 г. начато клиническое исследование препаратов Таксакад (паклитаксел) и Карбоплатин, в котором оценка безопасности и эффективности осуществляется в соответствии с международными критериями. Препараты Таксакад и Карбоплатин производятся ЗАО “Биокад” (Россия) из субстанций собственного производства на современном заводе, соответствующем стандартам надлежащей производственной практики GMP (Good Manufacturing Practice).
В данной работе представлены промежуточные результаты исследования по оценке безопасности и эффективности терапии Таксакадом и карбоплатином у больных НМРЛ III–IV стадий.
Материал и методы
Скрининговый отбор в открытое многоцентровое клиническое исследование прошли 47 пациентов 8 центров.
Для включения в исследование пациент должен был соответствовать следующим критериям:
- возраст не менее 18 лет;
- предоставление письменного информированного согласия;
- подтвержденный диагноз НМРЛ III–IV стадий;
- наличие измеряемых очагов заболевания;
- значение индекса ECOG (Eastern Oncology Cooperative Group) 0–1 балл включительно;
- ожидаемая продолжительность жизни более 20 недель;
- уровень билирубина менее 25,7, креатинина – менее 133,0 мкмоль/л, трансаминаз – не выше трехкратных максимальных нормальных значений (при наличии метастазов в печени – не выше пятикратных).
Пациент не подлежал включению в исследование в следующих случаях:
- проведение цитостатической терапии до включения в исследование;
- нейтропения;
- тромбоцитопения;
- наличие признаков периферической нейропатии второй и более высокой степеней;
- метастатическое поражение головного мозга;
- наличие заболеваний, способных повлиять на оценку выраженности симптомов основного заболевания;
- наличие других злокачественных заболеваний, отмеченных за последние 5 лет;
- наличие признаков хронической почечной недостаточности;
- наличие признаков эпилепсии или судорожного синдрома иного генеза в анамнезе;
- любые острые или активные хронические инфекции;
- любые признаки психического заболевания;
- наличие аллергии к веществам, входящим в состав исследуемых препаратов (Таксакад, Карбоплатин) в анамнезе;
- наркомания, алкоголизм, наличие ВИЧ-инфекции;
- одновременное участие в иных клинических исследованиях;
- наличие препятствий для введения химиопрепаратов в виде внутривенных инфузий.
В связи с несоответствием критериям включения два пациента выбыли на скрининге. Один больной выбыл до введения исследуемых препаратов вследствие развития острого инфаркта миокарда. Один пациент отозвал свое согласие на участие в исследовании до введения исследуемых препаратов. В течение 2 недель после включения в исследование у одного больного были выявлены метастазы в головной мозг, у двух – клинические признаки быстрого прогрессирования заболевания, что было расценено как несоответствие критериям включения и повлекло за собой прекращение участия в исследовании.
Таким образом, в анализ были включены 40 пациентов (31 мужчина и 9 женщин), соответствовавших критериям включения в исследование, и им по меньшей мере один раз ввели препараты Таксакад и Карбоплатин. Средний возраст пациентов составил 58 лет (от 34 до 74 лет).
В данном исследовании применяли Таксакад и Карбоплатин, фармацевтически эквивалентные оригинальным препаратам. В первый день каждого цикла больные, включенные в исследование, получали препарат Таксакад в дозе 175 мг/м2 в виде 3-часовой внутривенной инфузии после стандартной премедикации, затем проводилась 30-минутная внутривенная инфузия препарата Карбоплатин в дозе, соответствующей AUC = 6. Циклы повторяли каждый 21 день.
При развитии нейтропении III–IV степеней и/или фебрильной нейтропении после проведения химиотерапии назначали курс ежедневных подкожных инъекций препарата гранулоцитарного колониестимулирующего фактора (Г-КСФ) Лейкостима (филграстим, Биокад, Россия) в дозе 5 мкг/кг/ сут до достижения числа нейтрофилов > 2 × 109/л.
Оценку противоопухолевого эффекта проводили на основании критериев RECIST (Response Evaluation Criteria in Solid Tumors) после 3-го и 6-го курсов химиотерапии. Дальнейшая оценка эффективности терапии у этих пациентов осуществлялась через 6, 9 и 12 месяцев после начала лечения.
Первым шагом на этапе статистической обработки являлось определение типа имеющихся признаков и характера их распределения. Для описания количественных непрерывных признаков, распределение которых соответствовало закону нормального распределения, использованы среднее значение (М), среднее квадратическое отклонение (СКО) и/или 95 % доверительный интервал (ДИ). Для описания качественных порядковых признаков использованы медиана (Me) и интерквартильный размах (25–75 %).
Результаты
У 20,0 % больных диагностирована IIIа стадия НМРЛ, у 12,5 % – IIIb, у большинства (65,0 %) пациентов – IV стадия. У большинства больных были диагностированы множественные отдаленные метастазы: в плевру – 3 случая, печень – 4, надпочечники – 5, почки – 2, кости и позвоночник – 6, в отдаленные лимфатические узлы – 5; поражение другого легкого имело место в 12 случаев, метастазы в ипсилатерательную долю – в 7.
Было проведено от 1 до 6 циклов химиотерапии (1 курс – 2 пациентам, 2 курса – 3, 3 курса – 12, 4 курса – 1, 5 курсов – 3, 6 курсов – 19; всего – 177 курсов). Данные о безопасности проанализированы у всех больных, получивших хотя бы одну дозу исследуемого препарата.
Оценка безопасности и переносимости препаратов Таксакад и Карбоплатин основывалась на данных о частоте и выраженности побочных эффектов химиотерапии, в т. ч. отклонений показателей клинического и биохимического анализа крови (согласно критериям NCI CTC – National Cancer Institute-Common Toxicity Criteria, версия 2.0.), результатах оценки общесоматического статуса по шкале Карновского в модификации ECOG и оценки неврологического статуса. Данные по оценке токсичности на основании лабораторных показателей представлены в табл. 1.
Таблица 1. Общая токсичность (доля пациентов, у которых наблюдались данные изменения).
В течение всего периода наблюдения анемия III степени наблюдалась у 5 % пациентов, случаев анемии IV степени не было. Отмечено транзиторное снижение числа лейкоцитов до уровня, соответствующего I–II степеням токсичности у 60 % пациентов. Только у 10 % больных наблюдалась лейкопения III степени. Случаев лейкопении IV степени отмечено не было. Наблюдалось транзиторное снижение числа нейтрофилов до уровня I–II степеней токсичности у 35 % пациентов и у 40 % больных отмечена нейтропения III–IV степеней. При этом только в 35 % случаев потребовалось введение Г-КСФ. Доля пациентов, у которых наблюдалась нейтропения III–IV степеней, достигала 29,2 % к 4-му курсу химиотерапии (рис. 1). Тромбоцитопения III степени (без клинических проявлений) зарегистрирована в 2,5 % случаев. Не было отмечено случаев тяжелой тромбоцитопении IV степени.
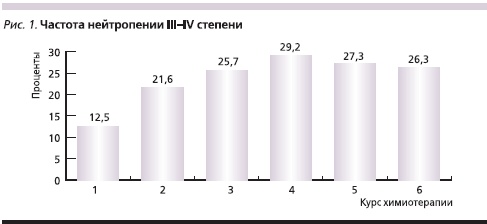
Лабораторные исследования не выявили ни одного случая развития выраженной печеночно-почечной токсичности. Для оценки степени тяжести периферической нейропатии – одного из наиболее часто встречающихся при применении паклитаксела и карбоплатина побочных эффектов – также использованы критерии токсичности NCI CTC, версия 2.0. При скрининге (до начала лечения) признаки периферической нейропатии отсутствовали у всех пациентов. Суммарно за время наблюдения в 25 % случаев отмечено развитие нейропатии I–II степеней, случаев развития тяжелых форм (III–IV степеней) зарегистрировано не было.
Общесоматический статус оценен по шкале ECOG. При скрининге до начала лечения оценка по шкале ECOG соответствовала 0 баллов у 16 (40 %) пациентов и 1 балл у 24 (60 %). После проведения 3 курсов химиотерапии (64-й день) данные доступны по 35 пациентам. Оценка по шкале ECOG соответствовала 0 у 12 (34,3 %) пациентов, 1 у 19 (54,2 %), 2 – у 3 (8,6 %) и 3 – у 1 (2,9 %). После проведения 6 курсов химиотерапии (126-й день, данные доступны по 19 пациентам) оценка по шкале ECOG соответствовала 0 у 3 (15,8 %) пациентов, 1 – у 15 (78,9 %) и 3 – у 1 (5,3 %). Ухудшение общесоматического статуса по шкале ECOG было связано с прогрессированием основного заболевания или сопутствующей патологией.
Зарегистрирован один серьезный побочный эффект – кардиомиопатия на фоне анемии после 4 циклов химиотерапии, повлекшая за собой летальный исход. Связь с исследуемой терапией расценена исследователем как маловероятная.
Кроме того, наблюдались следующие побочные эффекты (помимо описанных выше отклонений лабораторных показателей):
- у 50 % (n = 20) пациентов отмечена алопеция II–III степеней; связь с исследуемой терапией расценена как вероятная;
- у 17,5 % (n = 7) пациентов имели место случаи миалгии, артралгии и оссалгии легкой и средней степеней тяжести; расценены исследователями как связанные с приемом препарата;
- у 15 % (n = 6) пациентов были эпизоды астении легкой и средней степеней тяжести; расценены исследователями как связанные с приемом препарата;
- у 10 % пациентов (n = 4) отмечена тошнота легкой и средней степеней тяжести, иногда сопровождаемая рвотой; расценена исследователями как связанная с приемом препарата;
- у 10 % (n = 4) наблюдались аллергические реакции (эозинофилия, отек Квинке, реакции во время инфузии); расценены исследователями как связанные с приемом препарата. Два пациента выбыли из исследования в связи с развитием данных побочных эффектов;
- у 17,5 % (n = 7) пациентов отмечен болевой синдром различной локализации, который расценивался исследователями как не связанный с исследуемой терапией. Скорее всего боли были обусловлены проявлениями основного заболевания;
- у 10 % (n = 4) имели место одышка, кашель, кровохарканье, по мнению исследователя не связанные с приемом препарата;
- зарегистрирован 1 (2,5 %) случай двусторонней грибковой пневмонии. Расценен исследователями как не связанный с исследуемыми препаратами и не являющийся серьезным побочным эффектом, не оказавшим влияния на режим/дозу введения исследуемых препаратов. Пневмония полностью вылечена в результате антимикотической терапии;
- в 1 (2,5 %) случае наблюдалась лейкоцитурия через 20 дней от начала терапии. Расценена исследователями как не связанная с приемом препаратов. Полностью купирована антибактериальной терапией;
- в 1 (2,5 %) случае фебрильная температура наблюдалась еще до начала лечения и сохранялась в течение всего периода наблюдения. Расценена исследователями как несвязанная с исследуемой терапией.
Для оценки эффективности осуществлена компьютерная томография с контрастированием. Оценка эффективности проводимого лечения на основании критериев RECIST была выполнена у 35 пациентов, 3 больных не были включены в анализ эффективности, поскольку выбыли до проведения первой оценки (2 – в связи с аллергическими реакциями, 1 – в связи с неподтвержденным инструментальными методами прогрессированием заболевания); двое не включены в анализ эффективности, поскольку были исключены из исследования после проведения 2 курсов химиотерапии в связи с клиническим ухудшением, несмотря на то что при оценке по критериям RECIST отмечена стабилизация.
Из исследования выбыли 8 пациентов в связи с верифицированным прогрессированием заболевания после 2–3 циклов химиотерапии (у 1 из 8 больных с прогрессированием заболевания оценка была осуществлена после 2 курсов химиотерапии ввиду необходимости подтверждения клинических признаков прогрессирования), 2 пациентам со значительным регрессом опухоли (стабилизацией и частичным ответом) после 3 курсов химиотерапии были выполнены радикальные оперативные вмешательства, 7 больных выбыли до проведения повторной оценки эффективности (2 – ввиду развития побочных эффектов, 3 – по причинам, не связанным с исследуемым препаратом). У 20 пациентов проведена повторная оценка эффективности в соответствии с критериями RECIST (у 19 – после 6, у 1 – после 5 курсов химиотерапии).
В соответствии с критериями RECIST частичный ответ наблюдался у 7 (20,0 %) пациентов, стабилизация – у 20 (57,1 %) и прогрессирование – у 8 (22,9 %) пациентов.
Обсуждение
В соответствии с международным опытом регистрации воспроизведенных лекарственных средств на основе действующих веществ химической природы они считаются терапевтически эквивалентными оригинальным препаратам при наличии доказательств их фармацевтической эквивалентности и биоэквивалентности. Фармацевтическая эквивалентность подразумевает идентичность содержания действующего вещества и характеристик лекарственной формы оригинального и воспроизведенного лекарственных средств, а также соответствие воспроизведенного препарата фармакопейным критериям качества. Генерик считается биоэквивалентным оригинальному препарату в том случае, если его биодоступность (скорость и степень, с которой действующее вещество всасывается и появляется в большом круге кровообращения) не имеет существенных отличий от оригинального препарата. Исследование биоэквивалентности не может быть применено в случае внутривенно вводимых лекарственных средств, поскольку при внутривенном введении биодоступность всегда равна 100 %. Вследствие этого воспроизведенные лекарственные средства, вводимые внутривенно, в т. ч. большинство цитостатиков, разрешаются к медицинскому применению на основании лишь доказательств фармацевтической эквивалентности, получаемых в ходе государственной экспертизы качества.
Однако наилучшим доказательством терапевтической эквивалентности были и остаются клинические исследования, позволяющие оценить безопасность и эффективность лекарственного препарата. С учетом того, что комбинация паклитаксела и карбоплатина при НМРЛ хорошо изучена, объем клинического исследования воспроизведенных препаратов может быть ограниченным. Так, при проведении клинического исследования генериков по протоколам, дизайн которых повторяет ранее проведенные международные многоцентровые исследования, возможно сравнение полученных результатов с ретроспективными данными, а объем выборки может быть значительно сокращен.
Ввиду высокой актуальности вопроса импортозамещения дорогостоящих лекарственных средств более доступными отечественными аналогами и с учетом международного опыта исследования воспроизведенных препаратов нами был разработан протокол клинического исследования препаратов Таксакад и Карбоплатин, предварительные результаты которого представлены в данной работе.
С целью сравнительного анализа данных о частоте развития и выраженности побочных эффектов нами проанализированы литературные данные [3, 4, 5, 8, 10] о наиболее типичных побочных эффектах у пациентов, получивших от 6 до 10 циклов комбинированной химиотерапии препаратами паклитаксел и карбоплатин (табл. 2). Кроме того, в редких случаях было отмечено развитие тяжелой кардиотоксичности: от 0,5 % [4] и до 3,0 % в исследовании ECOG [10]; стоматита [5]; ототоксичности, в т. ч. тяжелой, в 3 % случаев [8].
Таблица 2. Профиль безопасности и токсичности комбинации паклитаксела и карбоплатина,по данным зарубежных клинических исследований.
Сравнительные данные по частоте побочных эффектов в данном и международных клинических исследованиях [5, 8] представлены на рис. 2. Данные о тяжелой (соответствующей токсичности III–IV степеней, согласно критериям NCI CTC, версия 2.0) гематологической токсичности представлены на рис. 3 [5, 8].


Таким образом, побочные эффекты, наблюдавшиеся при применении препаратов Таксакад и Карбоплатин (алопеция, тошнота, артралгии/миалгии, астения, нейтропения), являются ожидаемыми. Частота и выраженность побочных эффектов не превышали показатели, зарегистрированные в международных многоцентровых клинических исследованиях. Более того, частота некоторых побочных эффектов в этом исследовании была ниже по сравнению с литературными данными, что может быть связано с меньшей длительностью лечения, более низкой курсовой дозой химиопрепаратов и меньшим числом пациентов, включенных в исследование на текущий момент.
С целью оценки эффективности препаратов Таксакад и Карбоплатин нами проведено сравнение результатов оценки эффективности по критериям RECIST с данными литературы о частоте объективного ответа и стабилизации при использовании аналогичных схем химиотерапии в лечении распространенного НМРЛ. Так, в исследовании Kosmidis P. и соавт. [5] использование паклитаксела в дозе 175 мг/м2 (в виде 3-часовой внутривенной инфузии) и карбоплатина в дозе, соответствующей AUC = 6, каждые 3 недели позволило достичь полного ответа в 6,7 % случаев, частичного – в 18,9 %, стабилизации – в 38,9 % [5].
В многоцентровом рандомизированном сравнительном клиническом исследовании III фазы [8] при использовании схемы: паклитаксел в дозе 200 мг/м2 в виде 3-часовой внутривенной инфузии и карбоплатин в дозе, соответствующей AUC = 6, с интервалом 21 день, полный ответ достигнут у 1 % пациентов, частичный – у 24 %, стабилизация – у 40 %.
В сравнительном рандомизированном клиническом исследовании, проведенном ECOG [10], в одной из групп использовалась схема: паклитаксел в дозе 225 мг/м2 в виде 3-часовой внутривенной инфузии и карбоплатин в дозе, соответствующей AUC = 6, с интервалом 21 день. Доля пациентов, у которых достигнут полный ответ, составила менее 1%, частичный – 19 %, стабилизация – 21 %. В исследовании Scagliotti G. и соавт. [9] была проведена сравнительная оценка эффективности трех платиносодержащих режимов. В 1 (n = 204) из групп больные получали паклитаксел 225 мг/м2 и карбоплатин AUC = 6 в 1-й день каждые 3 недели. По результатам исследования частота достижения объективного эффекта и стабилизации в описываемой группе составила 68,5 %. В сравнительном рандомизированном исследовании III фазы, проведенном Southwest Oncology Group Trial [3], в одной из групп использована аналогичная схема. После проведения 6–10 циклов химиотерапии полный ответ зарегистрирован в 1 % случаев, частичный – в 24 %, стабилизация – в 33 %.
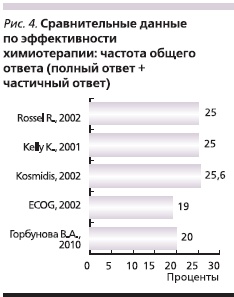
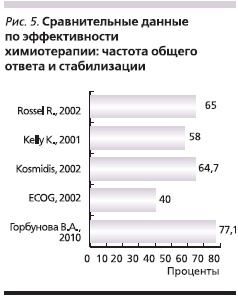
Результаты предварительной оценки эффективности применения Таксакада и карбоплатина по частоте достижения общего ответа (полный и частичный ответ; рис. 4), а также объективного эффекта и стабилизации (рис. 5) согласуются с данными международных многоцентровых клинических исследований.
Заключение
Таким образом, результаты данного исследования позволяют говорить о том, что безопасность и эффективность комбинированной химиотерапии на основе воспроизведенных препаратов Таксакад и Карбоплатин не имеют существенных различий по сравнению с безопасностью и эффективностью аналогичной схемы на основе оригинальных препаратов паклитаксела и карбоплатина.
Исследование эффективности и безопасности препаратов Таксакад и Карбоплатин в настоящее время продолжается. Целью второго этапа исследования является изучение препаратов в группе из 100 пациентов с определением уровня объективного клинического ответа, выживаемости, свободной от прогрессирования, и общей одногодичной выживаемости.
Необходимо отметить, что представленная работа является первым и пока единственным российским клиническим исследованием в области онкологии, оценивающим эффективность отечественных воспроизведенных препаратов в соответствии с критериями RECIST.
Информация об авторах:
Маренич Александр Федорович – старший научный сотрудник, РОНЦ им. Н.Н. Блохина РАМН.
Тел. 8 (495) 324-94-49;
Реутова Елена Валерьевна – РОНЦ им. Н.Н. Блохина РАМН.
Тел. 8 (495) 324-94-79;
Шевелева Людмила Петровна – заведующая отделением ХТ, Волгоградский областнойклинический онкологический диспансер № 1.
8 (442) 35-51-59;
Карасева Нина Алексеевна – кандидат медицинских наук, заведующая торакальным отделением,Городской онкологический диспансер, Санкт-Петербург.
Тел. 8 (812) 756-99-23;
Богданова Наталья Викторовна – заведующая ХТО поликлиники, Московский научно-исследовательскийонкологический институт им. П.А. Герцена Роcмедтехнологий;
Усков Дмитрий Альбертович – врач-онколог, Московский областной онкологический диспансер МЗ МО.
Тел. 8 (495) 521-58-38;
Хасанов Рустем Шамильевич – главный врач, Клинический онкологический диспансер, Казань.
Тел. 8 (843) 519-26-00;
Мухаметшина Гузель Зинуровна – Клинический онкологический диспансер, Казань;
Тузиков Сергей Александрович – доктор медицинских наук, руководитель отделения торакоабдоминальнойонкологии, НИИ онкологии Томского научного центра СО РАМН.
Тел. 8 (3822) 41-80-56;
Миллер Сергей Викторович – доктор медицинских наук, старший научный сотрудник отделения торакоабдоминальнойонкологии, НИИ онкологии Томского научного центра СО РАМН.
Хоринко Андрей Витальевич – заведующий 1-м ХТО, Пермский краевой онкологический диспансер.
Тел. 8 (342) 224-40-95



