Введение
В 2002 г. началась эпоха таргетной терапии немелкоклеточного рака легкого (НМРЛ), когда в клинической практике стали применяться ингибиторы EGFR [1, 2].
Второй мишенью для таргетной терапии при НМРЛ стал тирозин-киназный рецептор ALK, в 2007 г. впервые описанный в качестве возможной точки приложения [3]. В случае транслокации между 2-й и 5-й хромосомами происходит прикрепление гена ALK к гену EML4, что приводит к продукции измененного рецептора ALK, не требующего лиганда для его стимуляции.
Транслокация выявляется примерно в 5% случаев НМЛР. Такая опухоль чаще имеет строение аденокарциномы и встречается у молодых некурящих пациентов. Эти характеристики не имеют существенного отличия от таковых у пациентов с мутацией EGFR, хотя в сравнительном проспективном исследовании показано, что опухоли с транслокацией ALK чаще встречаются у мужчин и пациентов более молодого возраста [4].
Тестирование на наличие транслокации ALK входит в стандарт обследования пациентов с метастатическим НМЛР. Тестирование может быть проведено несколькими методами: FISH, ИГХ, ПЦР [5].
Первый ингибитор ALK – кризотиниб – был одобрен FDA в 2011 г. после подтверждения его эффективности во второй линии лечения. Позднее препарат изучался в качестве первой линии терапии в открытом рандомизированном исследовании PROFILE 1014, опубликованном в 2014 г. [6].
В настоящий момент в РФ одобрены 4 ALK-ингибитора: кризотиниб, церитиниб, алектиниб и лорлатиниб.
Лорлатиниб – препарат третьего поколения, отличается от остальных ингибиторов ALK своей структурой: макроциклическая молекула в отличие от ациклических предшественников. Лорлатиниб преодолевает резистентность, которая развивается вследствие мутаций на фоне использования препаратов предыдущих поколений (включая наиболее частую мутацию G1202R). Более того, наличие вторичных мутаций обусловливает чувствительность опухоли к лорлатинибу [7]. Также этот препарат имеет высокую способность проникать через гематоэнцефалический барьер (ГЭБ) и не является субстратом для таких транспортных молекул, как P-гликопротеин, и таким образом способен проникать через ГЭБ благодаря своей липофильной структуре [8–10]. Такие уникальные свойства молекулы обусловливают эффективность, демонстрируемую в клинических исследованиях, в особенности при поражении головного мозга (ГМ).
Лорлатиниб во 2-й и последующих линиях терапии
Лорлатиниб – это последний из одобренных FDA ALK-ингибиторов. Впервые одобрение FDA на использование препарата во второй и последующих линиях терапии ALK-позитивного НМРЛ получено в 2018 г. после опубликованного исследования II фазы [11].
В исследование были включены 276 пациентов с подтвержденной транслокацией ALK (228 пациентов) или ROS1 (47 пациентов), один пациент был исключен из анализа до начала лечения. Из них 30 пациентов не получали ранее какого-либо лечения, остальные составляли неоднородную группу, часть из которой получала таргетную терапию, часть – химиотерапию, остальные – как таргетную, так и химиотерапию. Более подробно группы пациентов представлены в табл. 1. Пациенты имели ECOG-статус 0–2, также допускалось наличие поражения ГМ. Препарат назначался в дозе 100 мг ежедневно, объективная оценка проводилась каждые 6 недель (каждые 2 курса терапии). Первичной конечной точкой была частота объективного ответа (ЧОО), вторичными – длительность эффекта, время до реализации эффекта, выживаемость без прогрессирования (ВБП), а также безопасность.
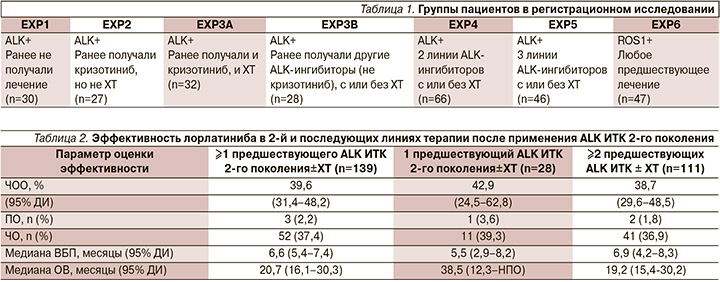
У пациентов, не получавших до этого лечения, ЧОО составила 90% (3% – полный ответ). Те пациенты, которые до включения в исследование получали только кризотиниб (с или без химитерапии), достигали ЧОО в 70% случаев (2% – полный ответ). Даже в группе, где испытуемые получали ранее 2 и более линий таргетной терапии (с/без химиотерапии), ЧОО достигли 39% больных (2% – полный ответ). В среднем ЧОО у пациентов, которые получили хотя бы одну линию терапии до включения в исследование, составила 47%, удалось обеспечить стабилизацию заболевания у 29% пациентов. Среди пациентов с поражением ГМ на момент включения в исследование ЧОО метастазов в ГМ была зарегистрирована у 63% пациентов (среди пациентов, ранее получавших только кризотиниб, – 87%), медиана длительности эффекта по метастазам в ГМ составила 14,5 месяцев. Эффективность лорлатиниба во 2-й и последующих линиях терапии после применения ALK ИТК 2-го поколения представлена в табл. 2.
Медиана времени до реализации эффекта во всех группах составила 1,4 месяца. Медиана длительности эффекта не была достигнута в общей группе при медиане времени наблюдения в 6,9 месяцев.
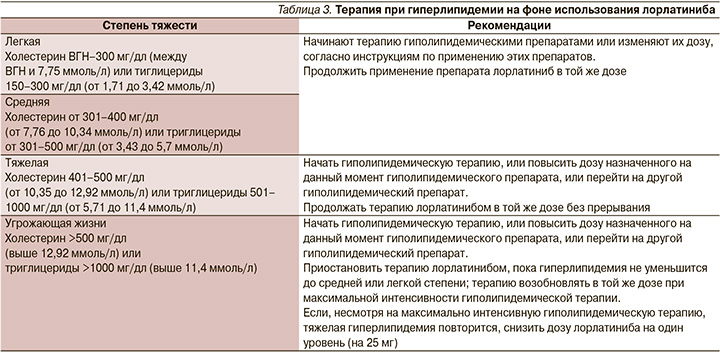
Наиболее частыми нежелательными явлениями (НЯ) были гиперхолестеринемия (81%), гипертриглицеридемия (60%), отеки (43%), периферическая полинейропатия (30%), увеличение веса (18%), когнитивные изменения (18%) и изменения настроения (16%). Большая часть всех НЯ оставалась на уровне 1–2-й степеней, самыми частыми НЯ 3-й степени были гиперхолестеринемия и гипертриглицеридемия (по 16%). Серьезные НЯ отмечены у 7% пациентов, самое частое из них (1%) – когнитивные нарушения. Прерывание лечения потребовалось 30% пациентов, снижение дозы – 22%. Наиболее частой причиной и для прерывания, и для снижения дозы были отеки. Отмена препарата потребовалась 3% пациентов, наиболее частая причина – когнитивные нарушения. Не было зарегистрировано ни одного НЯ 5-й степени. Стоит отметить, что НЯ, развивающиеся на фоне терапии лорлатинибом, на данный момент хорошо изучены и для управления ими разработаны и внедрены клинические рекомендации [12]. Рекомендуемый алгоритм действий при гиперлипидемии на фоне использования лорлатиниба представлен в табл. 3.
Обновленные результаты этого исследования, сфокусированные на эффективности лорлатиниба после использования ALK-ингибиторов второго поколения, были опубликованы в 2021 г. [13]. В общей сложности 139 пациентов до включения в исследование получили хотя бы одну линию терапии с использованием препаратов второго поколения (только 1-ю линию – 28 пациентов). Метастазы в ГМ в этой группе пациентов были у 68,3%, а также 48,2% получили лучевую терапию по поводу поражения ЦНС. Алектиниб как последнюю предшествующую терапию получали 44,6%, церитиниб – 33,8% больных.
ЧОО во всей исследуемой популяции составила 39,6%. Медиана длительности эффекта достигла 9,6 месяца, медиана ВБП – 6,6, медиана общей выживаемости – 20,7 месяца.
Однако среди тех пациентов, кто получил только одну предшествующую линию терапии, ЧОО была равна 42,9%. Медиана длительности эффекта – 6,2 месяца, медиана ВБП – 5,5, медиана общей выживаемости – 38,5 месяцев.
Измеряемые очаги в ГМ были зарегистрированы у 41% пациентов до начала лечения. Среди них ЧОО метастазов в ГМ составила 56,1% с 12 случаями полного ответа. При этом среди всех пациентов ЧОО по экстракраниальным проявлениям болезни составила 36,7% (5 случаев – полный ответ).
Эффективность препарата существенно не различалась в зависимости от того, какой ингибитор второго поколения был использован непосредственно до включения пациента в исследование.
Необходимо отметить проведенный молекулярно-генетический анализ плазмы и опухоли пациентов, включенных в настоящее исследование [14]. Результаты демонстрируют, что эффективность лорлатиниба была существенно выше у тех пациентов, кто к моменту начала терапии уже имел те или иные вторичные мутации в гене ALK после получения лечения ALK-ингибиторами второго поколения. Существенные различия были получены в отношении ЧОО в пользу пациентов, которые имели вторичные мутации: 62 против 32%, если учитывать результаты анализа плазмы крови, и 69 против 27%, если принимать во внимание результаты изучения опухолевой ткани. Таких различий не было у тех пациентов, которые получили перед началом исследуемой терапии только кризотиниб.
После использования ингибиторов ALK второго поколения у пациентов с появившимися мутациями в гене ALK увеличивалась не только ЧОО, но и выживаемость: медиана ВБП – 11 месяцев против 5,4, медиана длительности ответа на терапию – 24,4 месяца против 4,3. Отдельно были опубликованы результаты изучения изменения качества жизни пациентов на фоне терапии лорлатинибом [15]. Среди 255 пациентов, заполнивших по крайней мере два опросника, клинически значимое улучшение качества жизни отмечалось у 42,4% пациентов, стабилизация самочувствия – у 38%. Объективное ухудшение качества жизни отмечено в связи с ухудшением когнитивных способностей, периферической полинейропатии и алопеции. Значимое же улучшение качества жизни было связано с уменьшением слабости, бессонницы, кашля и боли.
Таким образом, лорлатиниб продемонстрировал свою высокую эффективность в отношении как экстракраниальных, так и интракраниальных метастазов после использования препаратов второго поколения. На основании результата представленного исследования лорлатиниб получил регистрацию как препарат для использования во второй и последующих линиях терапии пациентов с ALK-позитивным НМРЛ в 2018 г. от FDA, а в 2021 г. от Минздрава РФ.
Нельзя не упомянуть и результаты, полученные в реальной клинической практике. Так, с 2015 по 2019 г. во Франции был проведен объединенный анализ эффективности терапии лорлатинибом во второй и последующих линиях терапии [16]. Лечение получили 208 пациентов, из которых 77% имели поражение ГМ на момент включения в исследование. 79% пациентов получили лорлатиниб как 4-ю и последующую линию терапии. Медиана ВБП составила 9,9 месяца, ОВ – 32,9, медиана длительности терапии – 11,8 месяца. Частота объективных ответов достигла 49% (по метастазам в ГМ – 56%), а контроль над заболеванием был зарегистрирован у 86% пациентов. Токсичность в этом исследовании соответствовала регистрационному исследованию.
Собственный анализ проведен и опубликован и в Российской Федерации [17]. С 2017 по 2018 г. в него были включены 39 пациентов, из которых трое имели транслокацию ROS1, остальные – транслокацию ALK. До начала терапии всеми пациентами был использован кризотиниб и по крайней мере одна линия химиотерапии. ЧОО составила 71,7% (полная регрессия – 17,94%), стабилизация – у 25,6% пациентов. Таким образом, контроль над заболеванием получен у 97,3% пациентов. У пациентов с поражением ГМ ЧОО по интракраниальным очагам достигла 74%, при этом длительность терапии пациентов с поражением ГМ оказалась даже выше, чем без него. Профиль токсичности отвечал данным, ранее полученным в исследованиях.
Лорлатиниб в 1-й линии терапии
С учетом продемонстрированной в изложенном выше исследовании эффективности препарата в первой линии терапии было проведено открытое рандомизированное международное исследование III фазы CROWN [18].
В исследование были включены 296 пациентов с НМРЛ и подтвержденной транслокацией ALK, до этого не получавших системной терапии и имевших по крайней мере один измеряемый экстракраниальный очаг. Поражение ГМ являлось одним из факторов стратификации наряду с этнической принадлежностью пациентов. Первичной конечной точкой исследования была ВБП, по оценке заслепленной центральной команды. Вторичными конечными точками были ВБП, по мнению исследователя, ЧОО, интракраниальная ЧОО, время до прогрессирования метастазов в ГМ, длительность ответа, включая длительность контроля метастазов в ГМ и безопасность. Пациенты были рандомизированы в две группы: одна получала кризотиниб в дозе 250 мг в сутки, другая – лорлатиниб 100 мг в сутки. Контрольное обследование проводилось каждые 8 недель.
По результатам последнего анализа [19], опубликованного в декабре 2022 г., медиана ВБП в исследуемой группе не была достигнута, в контрольной группе составила 9,3 месяца. Трехлетняя ВБП составила 63,5 и 18,9% соответственно, риск прогрессирования снизился на 73%. ВБП, по независимой оценке, среди всех рандомизированных пациентов представлена на рисунке.
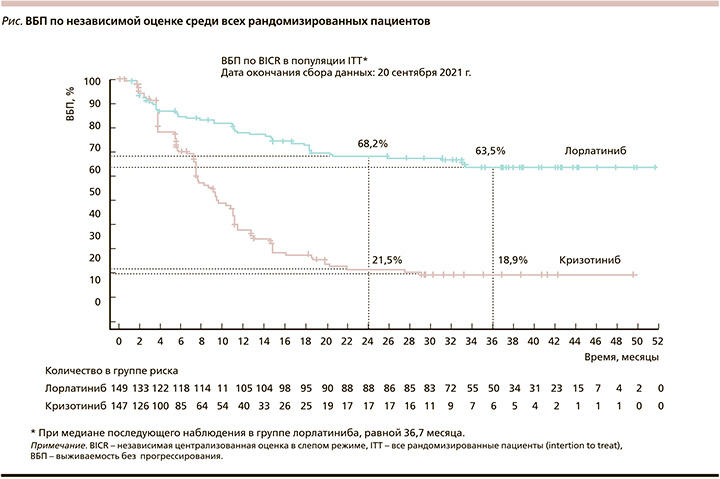
В подгруппе пациентов с изначальным поражением ГМ медиана ВБП в группе лорлатиниба не была достигнута, в группе кризотиниба равнялась 7,2 месяца. Отношение рисков прогрессирования метастазов в ГМ у пациентов с его исходным поражением составило 0,08 в пользу лорлатиниба, т.е. риск прогрессирования снизился на 92%.
У пациентов без метастазов в ГМ трехлетняя выживаемость без интракраниальной прогрессии составила 99 и 50%, опять же различия в пользу исследуемого препарата. Таким образом, риск интракраниальной прогрессии снижался на 98%. ЧОО в исследуемой группе составила 77% (3% – полный ответ), в контрольной – 59% (случаев полного ответа не зарегистрировано). ЧОО по интракраниальному ответу составила 65%, при этом частота полного интракраниального ответа – 59,5%.
Оценка качества жизни пациентов в обеих группах продемонстрировала, что в отношении практически всех аспектов (физических, ролевых, эмоциональных и социальных) кризотиниб уступал лорлатинибу, однако опережал в тех сферах жизни, которые касались когнитивной функции [20].
Профиль токсичности соответствовал полученному в более ранних исследованиях. Прерывание дозы потребовалось 49% пациентов в группе лорлатиниба, 47% в группе кризотиниба, снижение дозы препарата потребовалось в 21 и 15% случаев соответственно. Снижение дозы не вело к снижению эффективности терапии, ВБП к 12 месяцам составила 93% (без редукции дозы) и 89% (редукция дозы). НЯ привели к полному прекращению лечения 7% пациентов в исследуемой группе и 9% в контрольной.
В июне 2022 г. препарат одобрен Минздравом РФ для использования в первой линии пациентами с ALK-позитивным НМРЛ.
Клинические рекомендации
Согласно рекомендациям NCCN (v03.2023), лорлатиниб может быть использован как во второй и последующих линиях терапии, так и в первой линии терапии ALK-позитивного НМРЛ (наряду с алектинибом). Использование кризотиниба в первой линии, согласно авторам рекомендаций, возможно лишь при «определенных обстоятельствах» [21].
Рекомендации ESMO включают использование лорлатиниба в первой линии терапии. В случае если препарат используется после других ALK-ингибиторов, необходимо предварительное применение по крайней мере одного ингибитора, за исключением кризотиниба [22].
В рекомендациях Минздрава РФ, сформулированных АОР, использование лорлатиниба возможно при прогрессировании заболевания на фоне терапии алектинибом или церитинибом в первой линии, либо после применения кризотиниба и как минимум еще одного ингибитора ALK [23].
Клиническое наблюдение
Пациентка Х. 1950 года рождения, не курит и никогда не курила. В ноябре 2020 г. появились жалобы на периодический сухой кашель, одышку. По данным обследования (ПЭТ-КТ), выявлено опухолевое образование левого легкого, множественные метастазы в обоих легких, плевральный выпот слева, множественные метастазы в костях. В январе 2021 г. верифицирована ALK-позитивная аденокарцинома легкого.
Таким образом, установлен клинический диагноз «центральный рак верхней доли левого легкого T3N0M1. Метастазы в легких, костях, плеврит слева».
С февраля 2021 г. начато лечение в режиме алектиниб 600 мг×2 раза в сутки, per os.
На этом фоне отмечалась нарастающая положительная динамика в виде уменьшения образования левого легкого, регресса плеврального выпота. Исчезли жалобы, связанные с одышкой и кашлем.
Однако при контрольном обследовании (ПЭТ-КТ) в октябре 2022 г. зарегистрированы рост очага в легком, появление метастазов в лимфоузлах корня левого легкого и в надключичном лимфоузле, очага в теле левой подвздошной кости, нарастание левостороннего плеврального выпота. Таким образом, продолжительность терапии первой линии составила 20 месяцев.
С 25 ноября 2022 г. пациентка начала прием препарата лорлатиниб. На этом фоне, по данным ПЭТ-КТ, в марте 2023 г. отмечена положительная динамика: исчезновение накопления РФП в надключичном лимфоузле, уменьшение метаболической активности метастазов в костях, стабилизация остальных очагов.
Из НЯ отмечена триглицерилемия 1-й ст. и гиперхолестеринемия 2-й ст., не требовалось снижения дозы или прерывания приема препарата. Также не было отмечено изменения настроения или когнитивных функций. Субъективно пациентка не отмечала отрицательного изменения самочувствия на фоне терапии.
К настоящему моменту пациентка продолжает лечение, сохраняется удовлетворительное качество жизни, очередная оценка эффекта запланирована на июнь 2023 г.
Заключение
Лорлатиниб – это ALK-ингибитор третьего поколения, демонстрирующий существенную эффективность как у предлеченных, так и у ранее не получавших терапии пациентов, что подтверждается данными рандомизированных клинических исследований. Особенно впечатляющие результаты препарат демонстрирует при метастатическом поражении ГМ. В связи с отсутствием прямого сравнения лорлатиниба с препаратами второго поколения в первой линии лечения невозможно однозначно высказаться о преимуществе какого-либо из них по этому показанию. В то же время широкий спектр лекарственных препаратов увеличивает возможности лечения молодых и социально активных пациентов, страдающих ALK-позитивным НМРЛ.



