Введение
Псориаз – хроническое распространенное мультифакториальное иммуноопосредованное воспалительное заболевание кожи, характеризующееся повышенной скоростью деления кератиноцитов.
Наиболее эффективны для лечения псориаза системные биологические препараты. В то же время ряд нежелательных явлений, противопоказаний и высокая цена ограничивают их назначение [1].
Разработка новых препаратов, избирательно подавляющих специфичные для псориаза медиаторы воспаления на местном уровне, позволит избегать недостатков, присущих системной терапии [2].
В настоящее время одним из наиболее перспективных направлений терапии псориаза является подавление воспаления, опосредованного интерлейкином-36 (ИЛ-36). Данный цитокин – ведущий в патогенезе пустулезного псориаза. Кроме того, накапливается все больше сведений о его значении и для бляшечной формы заболевания [3, 4].
Необходимо отметить тот факт, что IL-36 в коже присутствует в неактивной форме. Для его активации необходимы сериновые протеазы нейтрофилов (катепсин G, нейтрофильная эластаза и протеиназа-3) [5]. Проведенные исследования показали, что в коже больных псориазом уровень сериновых протеаз значительно выше, чем у здоровых лиц, что позволяет рассматривать их в качестве мишеней для разработки таргетной терапии больных псориазом [6].
В Японии был синтезирован ингибитор сериновых протеаз – новый лекарственный препарат сивелестат (N-[2-[4-(2,2-диметилпропионилокси) фенилсульфониламино] бензоил]). Сивелестат применяется в ряде стран для системной терапии острой дыхательной недостаточности и цирроза печени, но для наружной терапии псориаза изучается впервые [7].
Цель исследования: оценка безопасности и переносимости сивелестата на лабораторных животных при его наружном применении.
Методы
Для определения безопасности препарата оценивали его острую токсичность, а для выявления переносимости – местно раздражающее, кожно-резорбтивное действия и аллергизирующие свойства.
Изучение острой токсичности
Изучение острой токсичности проводили, согласно методическим указаниям по изучению общетоксического действия фармакологических веществ.
Оценку острой токсичности проводили на 10 инбредных мышах линии BALB/с, 10 аутбредных мышах и 10 аутбредных крысах, которым наносили препарат в количестве 2000 мг/кг. Применялся наружный способ нанесения препарата, т.к. именно этот путь используется для изучения терапевтической эффективности действия сивелестата. После окончания периода карантина животных рандомизировали в две группы, волосы на коже спины однократно брили бритвой с острым лезвием, не допуская порезов. На побритый участок спины наносили крем (ланолин+масло оливковое+вода в равных долях), содержащий сивелестат в дозе 2000 мг/кг, что составляло от 40 до 400 мг в зависимости от вида и веса лабораторных животных.
Возможное проявление интоксикации оценивали в течение 2 недель при этом общее с учетом клинического состояния животных, поведения, приема корма и воды, координации движений, тонуса скелетных мышц, реакций на болевые, тактильные, звуковые и световые раздражители, частоты и глубины дыхательных движений, окраски слизистых оболочек и кожного покрова, положения хвоста, изменения массы тела. Причем в первый день наблюдение проводили каждый час – 12 часов. Результаты проведенного исследования заносили в таблицу.
Оценка местнораздражающего действия
Местнораздражающее действие препарата определяли методом эпикутанного действия на 10 инбредных и 10 аутбредных мышах (5 мышам каждой группы однократно наносили 5%-ный сивелестат и 5 – индифферентный крем). Оценку проводили сразу после однократного нанесения препарата, далее через 30 минут, 1, 2, 24, 48 часов. О болезненности судили по поведению и реакции животных при пальпации места нанесения. Обращали внимание на появление эритемы, шелушения, корок, трещин, пигментации.
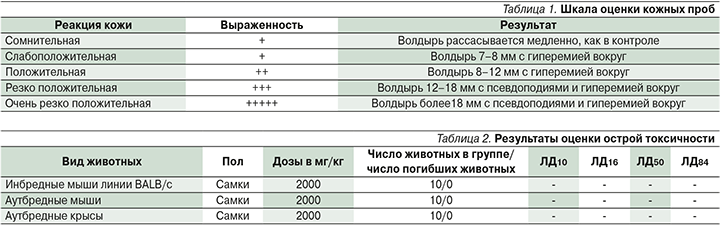
Реакцию кожи учитывали ежедневно по шкале оценки кожных проб С.В. Суворова:
- 0 баллов – отсутствие эритемы;
- 1 балл – очень слабое покраснение (розовый тон);
- 2 балла – видимое покраснение (розово-красный фон);
- 3 балла – покраснение от умеренного до сильного (красный тон);
- 4 балла – резко выраженная эритема (ярко-красный тон) с образованием корочек.
Оценка аллергизирующих свойств лекарственного средства
Изучение аллергизирующих свойств исследуемого препарата проводили методом эпикутанной сенсибилизации на 10 инбредных и 10 аутбредных мышах (5 мышам каждой группы наносили 5%-ный сивелестат и 5 – индифферентный крем).
Первое тестирование проводили после 10 аппликаций. При отрицательном или сомнительном результате число аппликаций доводили до 20, после чего животных тестировали повторно. Вещество наносили на протяжении 2 недель по 5 раз в неделю. Реакцию кожи учитывали ежедневно по шкале оценки кожных проб (табл. 1).
Изучение кожно-резорбтивного действия
Кожно-резорбтивное действие препарата исследовали на 10 инбредных и 10 аутбредных мышах. Животные были разделены на две группы: контрольную (по 5 мышей) и опытную (по 5 мышей). Лабораторные животные опытной группы были зафиксированы с помощью удерживающего устройства рестрейнера, хвосты погружали на 2/3 длины в шприц, содержвший крем (вода, ланолин и подсолнечное масло в равных пропорциях) с 5%-ным сивелестатом. Животные контрольной группы были зафиксированы аналогичным методом, хвосты погружались на 2/3 длины в шприц, содержавший крем (вода, ланолин и подсолнечное масло в равных пропорциях) без сивелестата. Время экспозиции составляло 30 минут. Реакцию учитывали сразу после, в течение 30 минут, 1, 2, 3, 4 часов после окончания экспозиции препарата (см. рисунок).

В качестве показателей оценки кожно-резорбтивного действия использованы общее физиологическое состояние животных, изменение массы тела, состав периферической крови.
Результаты
Результаты определения острой токсичности
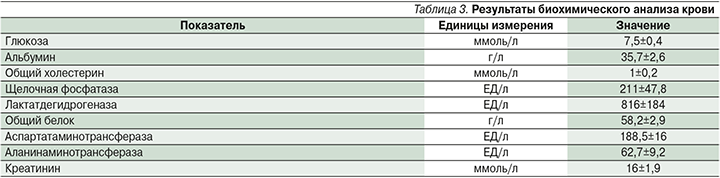
В ходе оценки острой токсичности при наружном однократном применении сивелестата в дозе 2000 мг/кг в течение 10 дней гибель животных отсутствовала, признаков интоксикации отмечено не было (табл. 2). Изменений в поведении, массе тела, приеме пищи, воды по сравнению с животными контрольной группы отмечено не было. Доза сивелестата была максимально возможной для нанесения на кожу животных – 2000 мг/кг.
При биохимическом анализе крови значимых изменений не обнаружено (табл. 3).
Результаты оценки местнораздражающего действия
При однократном нанесении на кожу лабораторных животных препарата изменений в общем состоянии и поведении отмечено не было. В месте нанесения препарата не наблюдалось эритемы, шелушения, утолщения кожной складки, пигментации (табл. 4). Через неделю выстриженные участки кожи покрылись волосами.
Результаты определения аллергизирующего действия
После проведения 10 аппликаций с препаратом сивелестат реакции кожи не обнаружено. Согласно методике, число аппликаций было увеличено до 20 и животных тестировали повторно. После проведения 20 аппликаций изменений на коже по шкале оценки кожных проб также не выявлено (табл. 5).
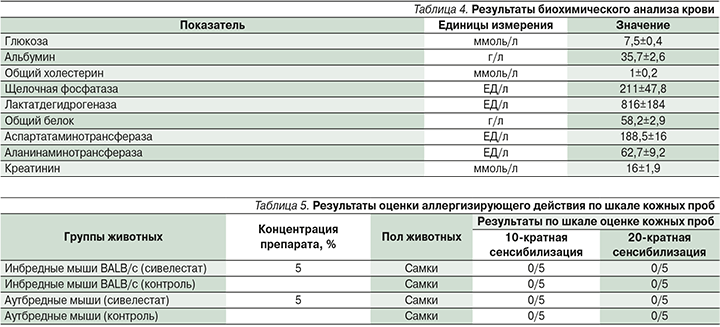
При проведении эпикутанной сенсибилизации на протяжении 2 недель реакции кожи по шкале оценки кожных проб не обнаружено, аллергизирующие свойства препарат на исследуемых животных не проявлял.
Результаты изучения кожно-резорбтивного действия
В эксперименте по изучению кожно-резорбтивного действия крема сивелестата не было выявлено выраженного влияния на кожу и каких-либо признаков интоксикации, смертельных исходов, изменений физиологического состояния, свидетельствующих о способности исследуемого препарата проникать в организм через неповрежденную кожу при однократном контакте с препаратом.
Обсуждение
Для оценки безопасности препарата проводили определение острой токсичности его действия, которое выполняли по общепринятой методике. На основании полученных результатов необнаружено изменений физиологического состояния лабораторных животных. Определение лабораторных показателей также не выявило значимых отклонений от нормальных значений, что в совокупности свидетельствует о безопасности данного препарата. Результаты проведенных трех фаз клинических исследований с участием человека и разрешение к системному применению данного препарата в ряде стран также свидетельствуют в пользу безопасности данного препарата.
Для определения переносимости крема сивелестата мы использовали оценку местно раздражающего, аллергизирующего и кожно-резорбтивного действий. Результаты проведенных исследований показали отсутствие значимых изменений на коже в месте нанесения препарата, а также общем состоянии экспериментальных животных. Таким образом, можно сделать вывод о хорошей переносимости сивелестата крема при его наружном применении.
Заключение
На основании результатов оценки острой токсичности, местно раздражающего, кожно-резорбтивного действий и аллергизирующих свойств на лабораторных животных установлен благоприятный профиль безопасности и переносимости сивелестата в наружной лекарственной форме.
Результаты проведенного исследования позволяют проводить дальнейшие этапы доклинических, а также клинических исследований с участием человека для определения безопасности и эффективности действия изучаемого препарата.
Вклад авторов. Жуков А.С. – написание текста. Жарун Е.Р. – проведение экспериментального исследования и обзора литературы. Чайкина М.А. – проведение экспериментального исследования и расчет результатов исследования. Хайрутдинов В.Р. – статистическая обработка данных. Самцов А.В. – концепция исследования. Красавин М.Ю. – редактирование. Гарабаджиу А.В. – дизайн исследования.
Финансирование. Исследование и публикации статьи осуществлены на личные средства авторского коллектива.



