Введение
Псориаз – распространенное хроническое мультифакториальное иммуноопосредованное воспалительное заболевание кожи, характеризующееся повышенной скоростью деления кератиноцитов [1].
Одно из ключевых значений в патогенезе псориаза в настоящее время придают цитокину интерлейкину-36 (ИЛ-36) [2]. С одной стороны, он всегда присутствует в коже и действует непосредственно на кератиноциты.
С другой – для активации ИЛ-36 необходим протеолитический процессинг, возможный только при участии сериновых протеаз нейтрофилов. На основании этих сведений можно предположить, что инактивация сериновых протеаз приведет к подавлению ИЛ-36-опосредованного воспаления в коже и позволит добиться ремиссии заболевания у больных псориазом [3, 4].
Для проверки гипотезы о возможности местного подавления развития псориатического воспаления нами был создан наружный препарат – крем, содержащий ингибитор сериновых протеаз (сивелестат).
Сивелестат – селективный, обратимый конкурентный ингибитор нейтрофильной эластазы, не влияющий на функции других протеаз человека. При системном назначении сивелестата происходит значительное снижение уровня фактора некроза опухоли α и ИЛ-6 в сыворотке крови посредством ингибирования NF-kB клеточного механизма [5].
Цель исследования: оценка терапевтической эффективности различных концентраций топического ингибитора сериновых протеаз (крема сивелестата) на лабораторной модели псориаза.
Методы
Для сравнения эффективности различных концентраций cивелестата в подавлении воспалительной реакции в коже использовали крем (ланолин+масло оливковое+вода в равных долях), содержащий сивелестат в концентрации 0,5, 1 и 5% (табл. 1).
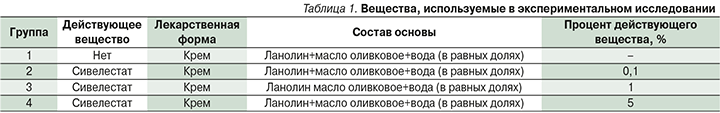
Работа проводилась на 40 инбредных мышах линии BALB/c, рандомизированных в 4 группы по 10 животных, согласно представленному дизайну (рис. 1).
Для проведения опыта предварительно сформировали экспериментальную имиквимод-индуцированную лабораторную модель псориаза, согласно патенту RU 2736000 C1. После окончания карантина лабораторных животных волосы на коже спины однократно брили бритвой с острым лезвием, не допуская порезов. Начиная со следующего дня на кожу спины равномерно наносили препарат имиквимод в количестве 62,5 мг 1 раз в день 10 дней.
Начиная с 6-го дня формирования модели через час после нанесения имиквимода мышам группы 1 (контрольная) наносили крем (ланолин+масло оливковое+вода в равных долях) 1 раз в день 5 дней, мышам группы 2 наносили крем (ланолин+масло оливковое+вода в равных долях), содержащий 0,1%-ный сивелестат 1 раз в день 5 дней, мышам группы 3 наносили крем (ланолин+масло оливковое+вода в равных долях), содержащий 1-ный сивелестат 1 раз в день 5 дней, мышам группы 4 наносили крем (ланолин+масло оливковое+вода в равных долях), содержащий 5-ный сивелестат 1 раз в день – 5 дней.
Для оценки терапевтической эффективности изучаемого препарата применяли клинический, гистологический и иммуногистохимический методы исследования.
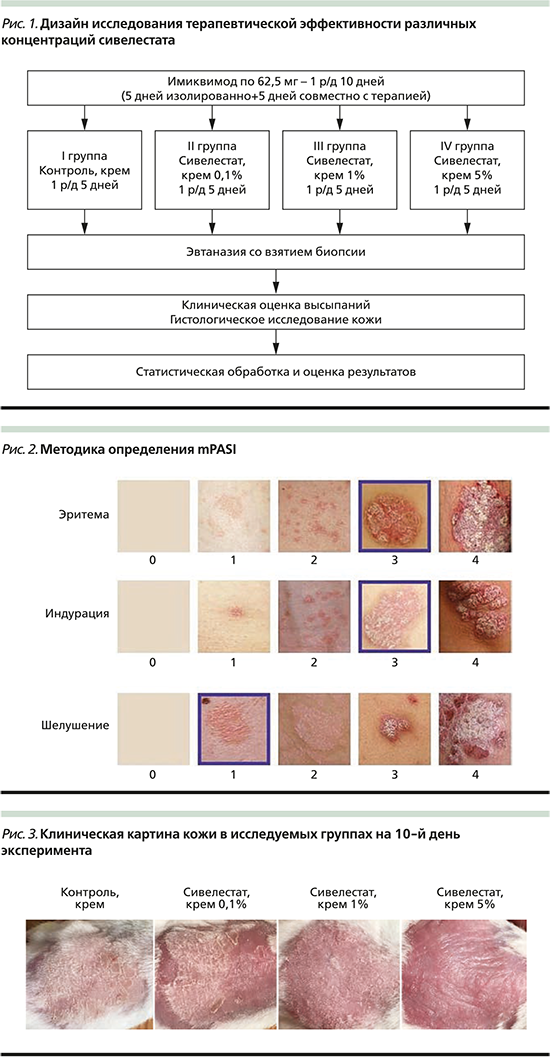
Ежедневно проводили клиническую оценку высыпаний на коже по PASI-модифицированному методу (mPASI), сходному с определением тяжести заболевания у больных псориазом. Оценивали показатели: эритема, индурация, шелушение по 5-балльной шкале от 0 до 4; распространенность высыпаний не считали, учитывая сходную их площадь (рис. 2). Затем показатели суммировали, получив индекс mPASI.
На 11-й день исследования всем мышам проводили эвтаназию передозировкой препарата Золетил® (производитель – Virbac Sante Animale, Франция), содержащий тилетамина гидрохлорид и золазепама гидрохлорид. Выполняли биопсию участка кожи спины. Из биоптатов готовили парафиновые блоки и срезы, согласно общепринятой методике. После этого выполняли гистологическое исследование, которое включило оценку толщины эпидермиса, выраженности акантоза, гиперкератоза, состава и количества воспалительного инфильтрата.
Выраженность гистологических изменений оценивали полуколичественно по 4-балльной шкале:
- «-» – отсутствие признака,
- «+» – слабовыраженный,
- «++» – средней выраженности,
- «+++» – выраженный признак.
Результаты исследования
В ходе индукции модели псориаза на 5-й день у лабораторных животных сформировался псориазиформный фенотип: эритема, шелушение, утолщение кожи. На 10-й день исследования в группах 1 (контроль) и 2 (0,1%-ный сивелестат), клинические изменения были ярко выражены, в группах 3 (1%-ный сивелестат) и 4 (5%-ный сивелестат) наблюдалось разрешение элементов кожной сыпи, более выраженное в последней группе (рис. 3).
Динамика клинических изменений до 6-го дня исследования во всех исследовательских группах была сходной. Начиная с 6-го дня в группах 3 и 4 наблюдалось снижение интенсивности эритемы, индурации и шелушения, в группах 1 и 2 интенсивность симптомов нарастала (рис. 4).
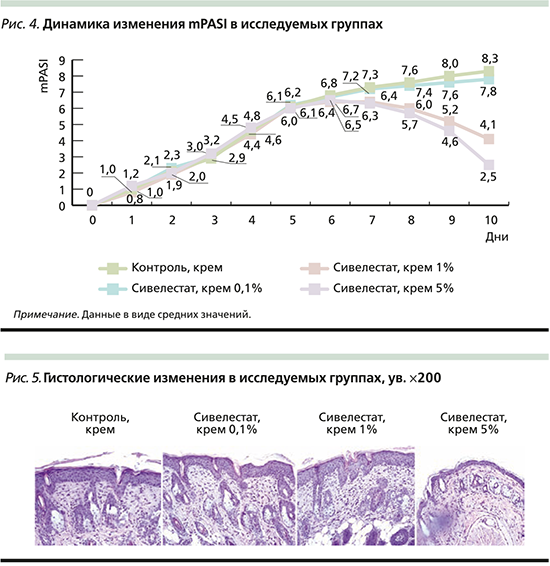
К 10-му дню исследования наиболее сильное снижение индекса mPASI наблюдалось в группах 4 (сивелестат 5%) и 3 (сивелестат 1%), значимо отличавшееся от показателей в группах 1 и 2 (p<0,05). При сравнении показателей индекс mPASI группы 4 значимо отличался от такового группы 3 (p<0,05). Группы 1 и 2 были сходными по данному значению (p>0,05). Индекс mPASI в группе 0,1%-ного крема сивелестат был ниже на 6%, 1%-ного – на 51%, 5%-ного сивелестата – на 70% по сравнению с контрольной группой (табл. 2).
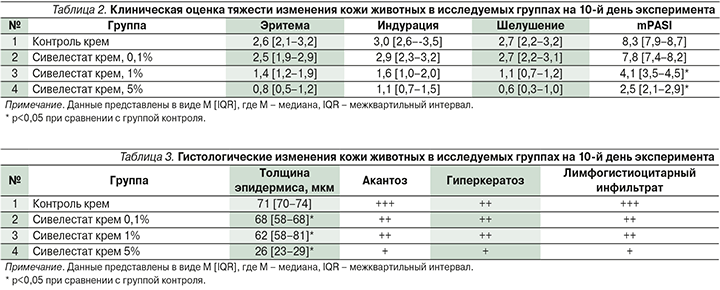
При оценке гистологических изменений к 10-му дню исследования в группах 1 (контроль) и 2 (1%-ный сивелестат) выявлены характерные для псориаза признаки: в эпидермисе – выраженный акантоз и паракератоз, в дерме – лимфогистиоцитарный инфильтрат и расширенные сосуды (рис. 5). В группе 3 (сивелестат 1%) гистологические изменения представлены в виде умеренного акантоза, фокального гиперкератоза и скудного лимфогистиоцитарного инфильтрата вокруг слаборасширенных сосудов сосочковой дермы. В группе 4 (сивелестат 5%) гистологические изменения были еще менее выражены (табл. 3).
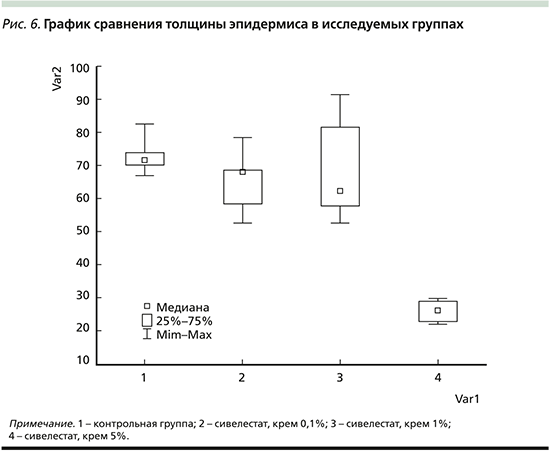
Установлено, что толщина эпидермиса группы животных 4 (сивелестат 5%) была в 1,0–2,7 раза меньше, чем в других группах (1, 2, 3; p<0,05 при попарных сравнениях с группой 4). Причем между собой группы 1 (контроль), 2 (сивелестат 0,1%) и 3 (сивелестат 1%) по толщине эпидермиса значимо не различались (рис. 6).
Обсуждение
В проведенной серии доклинических исследований нами уже была установлена безопасность и переносимость крема сивелестат при его наружном применении. Также показана терапевтическая эффективность применения 1%-ного крема сивелестата на лабораторной модели имиквимод-индуцированного псориаза. Задачей данного исследования стало определение терапевтической эффективности сивелестата в зависимости от концентрации используемого крема.
Результаты проведенного исследования показали, что препарат сивелестат крем в концентрациях 1 и 5% обладает выраженной терапевтической эффективностью на лабораторной модели имиквимод-индуцированного псориаза по сравнению с контрольной группой. Клиническое улучшение при использовании препарата сивелестата проявлялось в снижении интенсивности эритемы, шелушения и индурации кожи. Для объективизации результатов экспериментального исследования нами проведена оценка клинических изменений по индексу mPASI. Данный показатель был на 70% ниже в группе 5%-ного и на 51% – в группе 1%-ного крема сивелестата, что свидетельствует об их превосходстве над индифферентным кремом и 0,1%-ный кремом сивелестатом. Оценив силу разрешения клинических проявлений, установили почти 20%-ное преимущество крема сивелестата с концентрацией 5% над 1%.
Данные гистологического исследования кожи подтверждают результаты клинического обследования. В группах контроля и 0,1%-ного крема сивелестата наблюдались выраженные гистологические изменения, характерные для пораженной кожи больных псориазом. При применении 1%-ного крема сивелестата выраженность гистологических изменений резко снижалась, и наиболее слабые изменения были представлены в группе 5%-ного крема сивелестата. Толщина эпидермиса также была наименее выражена в группе 5%-ного крема сивелестата, а при использовании 0,1-, 1%-ного крема сивелестата и индифферентного крема различий не обнаружено.
Таким образом, нами показана зависимость выраженности терапевтической эффективности крема сивелестата от концентрации действующего вещества. Результаты проведенных доклинических исследований позволили установить силу противопсориатического действия препарата на основании комплексной оценки клинических и гистологических изменений. Необходимо проведение дальнейших клинических исследований для оценки действия крема сивелестата на больных псориазом.
Заключение
При наружном нанесении 5%-ного крема сивелестата 1 раз в день в течение 5 дней на лабораторной модели имиквимод-индуцированного псориаза наблюдается высокая терапевтическая эффективность по сравнению с группами плацебо, 0,1- и 1%-ного крема сивелестата. При использовании 5%-ного крема сивелестата выраженность клинических проявлений по mPASI была ниже на 70%, а гистологических по толщине эпидермиса ниже на 63%, чем в контрольной группе.



