Введение
Несмотря на высокую клиническую эффективность современных базисных противоастматических препаратов, применяемых в лечении бронхиальной астмы (БА), они не излечивают заболевание, поскольку не влияют на определяющие этиопатогенетические механизмы его формирования. Именно этим обстоятельством обусловлено возвращение симптомов болезни в течение небольшого промежутка времени после отмены базисной терапии большинству пациентов даже с нетяжелым течением БА. Сохраняющиеся проявления БА свидетельствуют о наличии хронического персистирующего воспаления, что диктует необходимость применения болезнь-модифицирующих методов лечения [1].
На современном этапе единственным болезнь-модифицирующим методом лечения БА остается аллерген-специфическая иммунотерапия (АСИТ). АСИТ способствует формированию клинико-иммунологической толерантности к этиотропным аллергенам и воздействует на все патогенетические звенья аллергического воспаления. Принципом действия АСИТ служит введение возрастающих доз того аллергена, к которому выявлена повышенная чувствительность и который отвечает за клинические проявления заболевания. Показано, что АСИТ предотвращает прогрессирование БА, препятствует переходу легких форм в более тяжелые, предупреждает развитие других видов сенсибилизации и позволяет уменьшать потребность в базисных лекарственных средствах (ЛС). В большинстве случаев по окончании АСИТ удается добиться многолетней ремиссии. Залогом успешного лечения БА данным методом считается приверженность пациентов терапии. Больные должны быть информированы о необходимости длительного проведения АСИТ, отсроченном времени начала действия метода, о возможности развития местных реакций. Коммуникация между врачом и пациентом повышает эффективность АСИТ, способствует контролю заболевания, обеспечивает комплаентность пациента и его удовлетворенность результатом лечения [2–7].
В практике наиболее распространены два варианта проведения АСИТ: подкожный и сублингвальный (СЛИТ), при этом наиболее перспективным является способ именно сублингвального введения аллергена, поскольку он характеризуется наиболее благоприятным профилем безопасности при равнозначной эффективности с подкожным методом. Лекарственные формы лечебных аллергенов для сублингвального применения представлены в виде раствора или таблеток [7, 8].
Немаловажным преимуществом СЛИТ является удобный для пациента и лечащего врача режим терапии, исключающий частые визиты и консультации. Данный метод особенно привлекателен для детей и их родителей. На современном этапе он является наиболее предпочтительным, поскольку характеризуется удобством, простотой и высокой эффективностью. В основе механизма действия СЛИТ лежит физиологическое свойство орального иммунного ответа с вовлечением толерогенных антигенпрезентирующих клеток. Обязательные условия для проведения СЛИТ: здоровая ротовая полость, отсутствие хронических воспалительных заболеваний полости рта и глотки.
Для лечения аллергических заболеваний, обусловленных сенсибилизацией к клещу домашней пыли (КДП; D. farinae и D. pteronyssinus), на российском фармацевтическом рынке имеется лечебная вакцина для СЛИТ «Аллерген клещей» компании Stallergenes (Франция) в форме раствора. В отношении данной лечебной формы аллергена накоплен немалый опыт как отечественными, так и зарубежными учеными [9].
Коронавирусная инфекция (КВИ) 2019 г. (COVID-19) в кратчайшие сроки стала настоящей пандемией. Несмотря на то что в настоящее время все основные усилия отечественного здравоохранения направлены именно на предотвращение распространения COVID-19 и организацию медицинской помощи больным, не следует забывать и о пациентах с хроническими заболеваниями, особую когорту среди которых составляют лица с аллергопатологией. Новые реалии заставляют нас рассматривать все вопросы диагностики, ведения и лечения аллергических заболеваний сквозь призму пандемии COVID-19. В связи с пандемией COVID-19 перед врачами практического звена здравоохранения встали сложные задачи относительно оказания плановой текущей помощи больным аллергопатологией, в т.ч. и касающиеся проведения АСИТ. Согласно имеющимся на сегодняшний день представлениям и опыту, стратегия проведения АСИТ больным БА в условиях пандемии должна корректироваться в соответствии с изменившимися условиями. В связи с этим ведущими российскими экспертами разработаны клинические рекомендации по ведению пациентов с аллергическими заболеваниями, получающими АСИТ в период пандемии COVID-19. Согласно этим рекомендациям, пациенты, АСИТ у которых проводится на фоне базисной терапии, не должны отменять базисную терапию, даже если у них есть симптомы COVID-19. Контроль над симптомами БА может обеспечиваться только соблюдением адекватного объема базисной терапии, поскольку при прекращении лечения существует риск развития обострений. Обострение БА может потребовать обращения в отделение неотложной помощи, что повысит риск инфицирования. В отношении АСИТ тактика врача несколько иная. В случае развития симптомов инфекции COVID-19 АСИТ следует прерывать и продолжать только после выздоровления пациента. Если пациент, получающий АСИТ, имел контакт с больным COVID-19, в этом случае необходимо принимать решение в зависимости от текущего состояния пациента. Данная рекомендация согласуется с общими положениями и инструкциями по проведению иммунотерапии аллергенами. Возобновление лечения аллергенами следует проводить обязательно под контролем врача-аллерголога-иммунолога. АСИТ следует инициировать только у бессимптомных и здоровых в отношении инфекции пациентов.
В условиях пандемии COVID-19 предпочтение следует отдавать именно сублингвальному введению аллергенов, поскольку данный метод не требует частых консультаций специалиста, что исключает риск инфицирования COVID-19 [8, 10, 11].
С начала пандемии COVID-19 БА как одному из хронических воспалительных аллергических заболеваний бронхолегочной системы уделяется пристальное внимание из-за поражения общего с новой КВИ органа-мишени. Изучение патогенетических механизмов влияния БА на риск заражения COVID-19, на развитие и исход инфекционного заболевания COVID-19 представляют огромный интерес. Важно понимание, какие больные БА наиболее подвержены риску заражения и тяжелого течения COVID-19. На данный момент накоплено немало данных, позволяющих пересмотреть первые теоретические представления, касающиеся особенностей течения и лечения БА на фоне заражения COVID-19. В начале эпидемии КВИ ведущие медицинские эксперты предполагали, что люди с БА, вероятнее всего, относятся к группе повышенного риска заражения и неблагоприятного течения COVID-19. Однако на сегодняшний день убедительных подтверждений этим опасениям не получено. Все больше появляется данных, согласно которым пациенты с аллергическими заболеваниями не подвержены большему риску развития COVID-19 и более серьезным осложнениям от инфекции, чем население в целом. В реальности на практике фиксируется весьма немного тяжелых обострений астмы при КВИ и не получено веских подтверждений, что БА – это значимый фактор тяжелого течения COVID-19. Возможно, исключением могут быть лишь более тяжелые случаи БА с недостаточным контролем состояния пациента [10, 12–14]. Более того, предполагается, что именно атопия и/или БА служат предиктором легкого течения инфекции COVID-19 [15–19].
Истинные причины нетяжелого течения инфекции COVID-19 у пациентов с аллергией окончательно пока еще не известны и требуют дальнейшего изучения. Предполагается, что определенную роль могут играть особенности цитокинового ответа, обусловленные аллергией, проведение базисной терапии ингаляционными и системными глюкокортикостероидами (ГКС), низкая экспрессия ангиотензинпревращающего фермента-2 (ACE2) и иные причины [10].
Известно, что системные ГКС, являясь иммунодепрессантами, могут способствовать развитию инфекций вирусно-бактериальной этиологии и влиять на их исход и течение. Однако на современном этапе большая часть пациентов БА получает безопасные в этом отношении ингаляционные ГКС (ИГКС). Пациенты, пренебрегающие базисной терапией ИГКС, имеют более высокие риски тяжелого обострения астмы. Проведенный мета-анализ исходов COVID-19 у пациентов с хроническими респираторными заболеваниями, получавших терапию ИГКС, показал, что в настоящее время нет убедительных и достаточных оснований для отмены пациентам с астмой базисного лечения этими препаратами. Однако требуется дальнейшее изучение этого вопроса [15, 20].
Известно, что определенное значение в патогенезе COVID-19 имеет взаимодействие вируса SARS-CoV-2 с ACE2 как с рецептором. Возможным объяснением наблюдения, что БА не является фактором риска неблагоприятного течения КВИ, служит снижение экспрессии гена ACE2 в клетках бронхолегочной системы, что и обеспечивает уменьшение восприимчивости к инфекции. Предполагается, что риск тяжелого течения COVID-19 у пациентов с астмой связан с его гендерной принадлежностью (мужской пол), афроамериканским происхождением и наличием сахарного диабета, поскольку у этих лиц выявлена повышенная экспрессия ACE2 [15–17]. Также значение, возможно, имеют курение и ожирение. Вероятно, лица с такими факторами и составляют определенную группу риска.
Таким образом, перед учеными стоит серьезная задача выявить ключевые предикторы и маркеры (демографические, клинические, лабораторные), которые помогут точно предсказать траекторию развития заболевания COVID-19, в т.ч. и у больных БА. Также важно оценить эффективность и безопасность проводимого лечения (базисного противоастматического и АСИТ) этой категории больных. На современном этапе пока имеется недостаточно цитируемых исследований, в которых представлена эффективность и безопасность АСИТ на фоне пандемии COVID-19.
Цель исследования: оценить клинико-иммунологическую эффективность и безопасность СЛИТ при лечении БА на фоне пандемии COVID-19.
Методы
Дизайн исследования – ретроспективное сравнительное открытое. Работа выполнена на кафедре аллергологии и иммунологии ПИУВ – филиала ФГБОУ ДПО РМАНПО Минздрава России и в клиниках ООО «МедМикс», ООО «МедМикс плюс».
В исследование включены 72 пациента с аллергической БА со средней степенью тяжести течения заболевания, из них 35 имели сочетанную патологию в виде аллергического ринита и БА. Этиопатогенез БА был обусловлен сенсибилизацией к КДП (D. farinae и D. pteronyssinus). Сенсибилизация к КДП была подтверждена результатами аллергологического анамнеза, скарификационных кожных тестов к D. farinae и D. pteronyssinus и специфических IgE-антител методом ImmunoСAP.
Диагноз БА устанавливался в соответствии с критериями, изложенными в Федеральных клинических рекомендациях и Международном руководстве GINA, 2020 [21].
В наблюдаемой группе мужчин было 28 (38,9%), женщин – 44 (61,1%). Средний возраст обследованных составлял 36,64±1,69 (34,00 [24,00; 50,00]) года. Длительность БА до начала лечения варьировалась от 1 года до 8 лет и составила в среднем 4,33±0,21 (4,35 [2,80; 5,40]) года.
Согласно дизайну исследования, все пациенты с аллергической БА были разделены на две группы: в 1-ю включены 42 человека, получавших СЛИТ препаратом Сталораль «Аллерген клещей» на фоне базисной противовоспалительной терапии, во 2-ю группу – 30 человек, получавших только стандартную базисную противовоспалительную терапию согласно степени тяжести. Группы были сопоставимыми по полу, возрасту, степени тяжести БА и результатам аллерго-иммунологического тестирования (р>0,05).
Все пациенты получали противовоспалительную терапию в соответствии с рекомендациями GINA [21]. В качестве базисной фармакотерапии использовались средние дозы ИГКС и короткодействующие β2-агонисты (КДБА) по требованию. Те же дозы больные получали перед включением в исследование. Для унифицированной оценки дозового режима ИГКС независимо от индивидуального использования препаратов их доза рассчитывалась в соответствии с терапевтической эквипотентностью по беклометазона дипропионату и составила в среднем перед началом АСИТ 751,19±15,00 (750,00 [700,00;850,00]) и 740,00±19,68 (750,00 [650,00;800,00]) мкг соответственно. В дальнейшем каждые 3 месяца доза базисных противоастматических ЛС пересматривалась, при сохранении контролируемого течения астмы снижалась.
Пациентам первой группы выполнялась СЛИТ этиотропным аллергеном. Иммунотерапия, сочетаемая с вышеуказанной фармакотерапией, проводилась в соответствии с международными стандартами по лечению аллергенами. Для лечения использовали лечебный препарат Сталораль «Аллерген клещей» производства Stallergenes (Франция), согласно инструкции по применению препарата. Пациенты принимали аллерген круглогодично сублингвально: начальный набор дозы (10 ИР/мл) осуществлялся с 1 до 5 нажатий, далее применялась доза 300 ИР/мл с 1 до 4 нажатий, в дальнейшем поддерживающий курс проводили дозой 300 ИР/мл в режиме 4 нажатий ежедневно в течение 3 лет. Коррекция дозы аллергена проводилась в соответствии с рекомендациями при обострении заболевания, пропусках, наличии побочных реакций [9, 22].
Всем пациентам проводились плановые осмотры аллерголога 1 раз в 3 месяца для оценки контроля течения БА, пересмотра базисной противоастматической терапии, оценки нежелательных явлений и переносимости СЛИТ.
Динамику течения БА оценивали по частоте и выраженности симптомов, необходимости применения КДБА, объему базисной терапии, толерантности к физической нагрузке и частоте обострений [21].
Эффективность СЛИТ в динамике оценивали в конце каждого года наблюдения, учитывая течение БА (изменение частоты и интенсивности клинических симптомов, количества обострений, объем базисной терапии и потребность в симптоматических КДБА, показатели спирометрии, уровень контроля БА). Результаты оценивались как отличные, если не отмечено обострений, клинические симптомы отсутствуют или выражены незначительно, значительно уменьшился объем применяемых ЛС, существенно повысился контроль БА; хорошие, если наблюдались редкие обострения, сохранение клинических симптомов меньшей интенсивности, уменьшение объема применяемых ЛС, повышение контроля БА; удовлетворительные, если наблюдалось сохранение частоты обострений, незначительное уменьшение выраженности клинических симптомов и потребности в ЛС, контроль БА прежний и неудовлетворительные, если клиническое течение БА осталось без изменений или ухудшилось.
Контроль уровня БА осуществляли при помощи опросника, где учитывались дневные и ночные симптомы, потребность в симптоматических препаратах и любое ограничение активности из-за БА. Для удобства статистической обработки использовали оценку в баллах: где полный контроль составил 1 балл, частичный – 2, отсутствие контроля – 3 балла.
Также использовали тест по контролю астмы (АСT-тест), где сумма 25 баллов означала полный контроль, 20–24 – не полный, но хороший контроль, сумма 16–19 баллов – частичный контроль, менее 15 – полное отсутствие контроля.
Оценку функции внешнего дыхания проводили при помощи спирометрии, согласно стандартной методике, на приборе MIR Spirolab 1 New.
Лабораторное исследование осуществляли в сетевой лаборатории «XELIX». Определение аsIgE к аллергенам D. farinae и D. pteronyssinus проводилось методом молекулярной диагностики ImmunoCAP (кЕдА/л). Исследование общего IgE (МЕ/мл) осуществляли методом иммуноферментного анализа.
Скарификационные кожные пробы выполнены стандартным методом с отечественными лечебно-диагностическими аллергенами D. farinae и D. pteronyssinus, согласно инструкции (ООО, Микроген).
Критерии включения в исследование: диагноз «аллергическая БА со сроком подтверждения более 1 года»; возраст больных от 18 до 65 лет; применение больными перед включением в исследование в течение последних 6 месяцев средних доз ИГКС в сочетании с КДБА; контролируемое или частично контролируемое течение БА с исходной оценкой по данным АСТ-теста по контролю над астмой не менее 19 баллов; гиперчувствительность к клещам домашней пыли (D. pteronissinus, D. farinae), подтвержденная наличием симптомов астмы при контакте с причинно-значимыми аллергенами, результатами аллергологического обследования, включающего наличие положительных результатов исследования asIgE и кожного тестирования с этиотропными аллергенами более 5 мм в диаметре (допускалась сопутствующая сенсибилизация к пыльцевым и/или эпидермальным аллергенам в отсутствие постоянного контакта с животными дома); информированное согласие испытуемых на проведение всех процедур исследования и СЛИТ в течение не менее 3 лет.
Исследование проведено с 2017 по 2021 г. С начала пандемии COVID-19 (с 2019 по 2021 г.) у пациентов с БА, получавших СЛИТ, помимо эффективности и безопасности вакцинации оценивался риск заражения COVID-19. В случае развития заболевания COVID-19 у наблюдаемых пациентов изучались тяжесть и исходы его течения, а также влияние КВИ на течение БА. В связи с пандемией COVID-19 и временным запретом очных визитов в клинику организована возможность консультаций с использованием телемедицинских технологий.
Статистическая обработка материала проводилась с применением общепринятых в медицине методов вариационной статистики с помощью пакета прикладных программ Statistica 6.0 на персональном компьютере. Критический уровень значимости при проверке статистических гипотез принят за р<0,05. Показатели были представлены в виде средней, медианы (Ме) и квартилей [Р25%; Р75%]. При сравнении изучаемых групп до и после терапии использовали тест Wilcocson.
Результаты
Наблюдение и оценка результатов лечения у больных БА, которым проводилась СЛИТ на фоне фармакотерапии, показал ее явный клинический эффект.
Важным параметром эффективности СЛИТ является снижение объема базисной фармакотерапии. Исходно пациенты обеих групп, включенные в исследование, несмотря на постоянную и длительную терапию средними дозами ИГКС (в среднем 751,19±15,00 (750,00 [700,00; 850,00]) и 740,00±19,68 (750,00 [650,00; 800,00] мкг соответственно по БДП), перед началом СЛИТ имели признаки недостаточно контролируемого течения заболевания. Применение СЛИТ в отношении больных первой группы сопровождалось планомерным снижением доз ИГКС к окончанию лечения, привело к значимому уменьшению объема противовоспалительной терапии. Мониторинг среднесуточных доз ИГКС базисной терапии показал положительную динамику в отношении этого важного параметра. На фоне СЛИТ средняя доза 751,19±15,00 (750,00 [700,00; 850,00]) у больных первой группы уменьшилась до низких значений – 465,48±18,31 (450,00 [350,00;550,00]) мкг (р<0,05), в то время как у больных второй группы не было изменений 740,00±19,68 (750,00 [650,00; 800,00]) и 780,00±16,19 (750,00 [750,00; 850,00]) мкг (р>0,05) соответственно по БДП. В целом же доза ИГКС на фоне 3-летней АСИТ снизилась по сравнению с исходным значением почти в 1,7 раза. Таким образом, согласно дизайну исследования, исходно все 42 пациента первой группы (100%) получали средние дозы ИГКС, следовательно, через 3 года наблюдения – 30 (71,4%) человек перешли на низкие среднесуточные дозы. Кроме того, 3 (7,1%) после окончания полного курса СЛИТ ИГКС удалось полностью отменить. В то же время у 2 (6,7%) пациентов 2-й группы отмечено повышение доз применяемых ИГКС до высоких. Необходимо отметить, что снижение дозы ИГКС не только не сопровождались ухудшением состояния больных, но и наблюдалось уменьшение частоты и выраженности дневных и ночных симптомов БА.
Одним из ключевых показателей эффективности СЛИТ является уровень контроля БА. Согласно оценке данных опросника после проведения СЛИТ, у больных повысился контроль над заболеванием. Уже в первый год лечения вакциной Сталораль «Аллерген клещей» была отмечена положительная динамика со стороны уровня контроля БА, которая имела устойчивый характер и сохранялась на протяжении всего времени исследования. У больных 1-й группы после проведения СЛИТ контроль повысился с 1,93±0,09 (2,00 [2,00;2,00]) до 1,12±0,05 (1,00 [1,00; 1,00]) балла (р<0,05), в то время как у пациентов 2-й группы, не получавших СЛИТ, контроль остался на прежнем уровне и составил 2,1±0,14 (2,00 [2,00; 2,00]) балла при исходном значении 2,03±0,09 (2,00 [2,00; 3,00]) балла (р>0,05).
Динамический анализ уровня контроля БА, проводимый с помощью АСТ-теста, также подтвердил эффективность СЛИТ в отношении влияния на течение астмы. Исходно у пациентов имелись признаки недостаточно контролируемого течения, о чем свидетельствовали результаты АСТ-теста, составившие в среднем для больных первой группы 22,60±0,17 (23,00 [22,00; 23,00]) балла, для больных второй группы 22,37±0,22 (22,50 [22,00; 23,00]) балла соответственно, при возможном максимальном значении в 25 баллов. Суммарный балл АСТ-теста значимо изменился на фоне СЛИТ препаратом Сталораль «Аллерген клещей». Так, к концу лечения у пациентов первой группы уровень контроля повысился с 22,60±0,17 (23,00 [22,00; 23,00]) до 24,36±0,11 (24,50 [24,00; 25,00]) балла, что статистически значимо выше (р<0,05). У пациентов второй группы такой тенденции отмечено не было, средние значения составили 22,57±0,23 (23,00 [22,00; 24,00]) балла, при исходном показателе 22,37±0,22 (22,50 [22,00; 23,00]) балла (р>0,05).
О повышении уровня контроля БА на фоне иммунотерапии, а также об эффективности лечения аллергенами свидетельствует и динамика частоты обострений заболевания. Так, если в течение года, предшествовавшего СЛИТ, количество обострений БА в первой группе больных составило в среднем 2,26±0,14 (2,00 [2,00; 3,00], то к концу лечения число обострений снизилось до 1,05±0,11 (1,00 [1,00; 2,00]; р<0,05). В отношении второй группы изменение количества обострений не было значимым. Частота обострений у пациентов второй группы до начала лечения составила 2,33±0,16 (2,00 [2,00; 3,00]) раза в год. После лечения данный параметр составил 2,10±0,18 (2,00 [1,00; 3,00]; р>0,05). Таким образом, в результате СЛИТ произошло снижение количества обострений в 2,1 раза, в то время как у больных, получавших только базисную противоастматическую фармакотерапию, этот показатель уменьшился в 1,1 раза (р<0,05).
Если до проведения СЛИТ необходимость стационарного лечения отмечалась в 17,2% обострений, то по окончании лечения госпитализация при обострении БА потребовалась только 5,2% больных. Кроме того, улучшилось клиническое течение сопутствовавшего аллергического ринита: 11 больным прекращена терапия интраназальными стероидами. Результаты работы свидетельствуют: СЛИТ изменяет течение сопутствующего аллергического ринита, на это указывают полученные данные об уменьшении использования базисных препаратов, повышении контроля и улучшении качества жизни.
Анализ показателей спирометрии по окончании лечебного периода СЛИТ больных первой группы не показал статистически значимых различий по объему форсированного выдоха за первую секунду (ОФВ1) по сравнению с исходными параметрами: 96,95±0,24 (97,00 [96,00; 98,00]) и 97,57±0,15 (97,50 [97,00; 98,00])% (р>0,05). У больных второй группы, несмотря на регулярную базисную терапию ИГКС, имелась тенденция к снижению данного показателя: 93,63±0,38 (94,00 [93,00; 95,00]) и 92,93±0,30 (93,00 [92,00; 94,00])% (р>0,05). Вероятно, полученный результат связан с включением в исследование больных с достаточно высокими спирометрическими показателями, что обусловлено базисной противоастматической фармакотерапией ГКС.
Таким образом, эффективность СЛИТ препаратом Сталораль «Аллерген клещей» с учетом отличных и хороших результатов за 1-й год составила 73,9%, за 2-й – 85,7%, за 3-й – 92,8% (табл. 1).
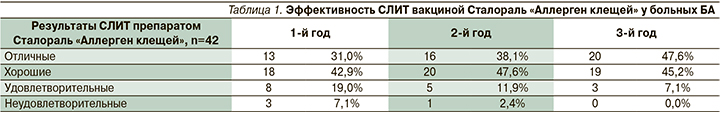
Для получения наиболее значимого благоприятного результата СЛИТ необходимо следовать инструкции по применению вакцины Сталораль «Аллерген клещей», согласно которой требуется длительность курса не менее 3 лет, поскольку АСИТ имеет дозозависимый эффект.
По завершении курса СЛИТ вакциной Сталораль «Аллерген клещей» больным БА проведена динамическая оценка ряда аллергоиммунологических параметров (табл. 2).
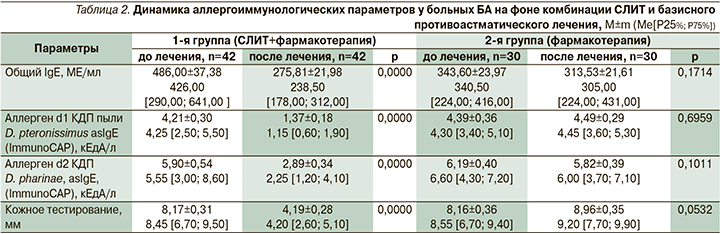
Мониторинг аллерго-иммунологических показателей на фоне СЛИТ (табл. 2) продемонстрировал значительную положительную динамику после проведенной терапии (р<0,05), что подтверждает снижение активности аллергического воспаления.
У пациентов первой группы произошло снижение значений общего IgЕ и asIgE к бытовым аллергенам, во второй группе показатели остались на прежнем уровне.
В первой группе выявлено снижение уровня общего IgЕ в 1,8 раза (р<0,05) – с 486,00 до 275,81 ME/мл, в то время как во второй отмечены лишь незначительные колебания средних значений (с 343,60 до 313,53 ME/мл; р>0,05).
На фоне СЛИТ уровень asIgE к клещу D. pteronissimus снизился у пациентов в 3,1 раза (р<0,05), с 4,21 до 1,37 кЕдА/л, в то время как у больных, получавших только базисное противоспалительное лечение, показатели остались на прежнем уровне (с 4,39 до 4,49 кЕдА/л; р>0,05). Аналогичная тенденция наблюдалась и в отношении asIgE к аллергену клеща D. pharinae.
У больных первой группы уровень asIgE к клещу D. pharinae снизился в 2 раза (р<0,05) – с 5,90 до 2,89 кЕдА/л, у пациентов второй группы – только с 6,19 до 5,82 кЕдА/л (р>0,05).
Полученные результаты согласуются с современными положениями в отношении маркеров эффективности СЛИТ [23, 24].
Имелась тенденция к уменьшению диаметра кожных проб с лечебно-диагностическими аллергенами D. farinae и D. рteronyssinus. В первой группе наблюдалась положительная динамика в отношении диаметра со снижением в 1,9 раза (с 8,17 до 4,19 мм; р<0,05), в то время как у пациентов второй группы, напротив, была тенденция к увеличению диаметра (с 8,16 до 8,96 мм; р>0,05).
Необходимо отметить, что лучшие результаты в отношении эффективности СЛИТ получены от пациентов с исходно большими показателями аsIgЕ и кожных проб. Таким образом, потенциальными кандидатами успешного проведения СЛИТ являются пациенты с высоким уровнем сенсибилизации к КДП по результатам специфического обследования.
Таким образом, анализ полученных данных показал, что СЛИТ оказывает болезнь-модифицирующее патогенетическое действие на больных аллергической БА.
Помимо эффективности СЛИТ важной характеристикой является ее переносимость и безопасность. В проведенном исследовании подтверждена высокая безопасность сублингвального метода иммунотерапии, зарегистрированы только единичные побочные реакции у 8 (19,0%) пациентов, которые носили локальный характер и проявлялись легким зудом с небольшой отечностью в подъязычной области, зудом в ушах. Все нежелательные реакции купировались самостоятельно и отмечались на этапе наращивания дозы. Ни в одном случае побочные эффекты не были причиной прекращения СЛИТ и не требовали внесения изменений в схему лечения. Двум больным в связи с этими симптомами были назначены симптоматически антигистаминные препараты второго поколения.
Согласно последним публикациям, БА не относится к категории заболеваний, усугубляющих прогноз при новой КВИ [25]. Результаты нашего наблюдения согласуются с этим фактом.
С целью обеспечения безопасности в связи с пандемией COVID-19 на базах лечебных учреждений, где проводилось исследование, организована помощь с использованием телемедицинских технологий. Также у пациентов была возможность связаться с лечащим врачом по телефону посредством приложений WhatsApp и Viber. Большинство пациентов отметили удобство и безопасность такого режима консультаций и выразили желание и в дальнейшем (в период отмены режима самоизоляции) пользоваться данной формой наблюдения.
Среди всех пациентов с БА случаи инфицирования и заболевания COVID-19 мы зафиксировали у 17 (23,6%) человек, при этом 10 из них получали СЛИТ. Половина заболевших пациентов получали третий курс лечения аллергеном. Важной задачей было своевременное проведение дифференциальной диагностики обострения БА и инфекции COVID-19. Всем заболевшим пациентам осуществлены срочные консультации аллерголога-иммунолога в режиме on-line. Дальнейшее наблюдение аллергологом проводилось в удаленном режиме до полного выздоровления. Непосредственное обследование и лечение инфекционного процесса у таких пациентов осуществлялись медицинским работником по месту жительства. При этом определенную диагностическую ценность в связи с невозможностью провести на этом этапе спирометрию ввиду возможного риска распространения инфекции имели индивидуальные пикфлоуметры. Показатели пикфлуометров были использованы также для телемониторинга контроля над БА. У всех пациентов инфекция COVID-19 протекала в легкой форме в виде ОРЗ. После верификации диагноза и установления степени тяжести состояния им продолжена базисная терапия ИГКС. Все пациенты были в обязательном порядке проинструктированы о рисках неблагоприятного исхода, обусловленных прекращением приема базисной терапии. В связи с инфицированием COVID-19 иммунотерапия пациентов была временно приостановлена до полного выздоровления и получения отрицательных мазков ПЦР к COVID-19. СЛИТ возобновлялась после полного выздоровления после консультации аллерголога, согласно инструкции. Среди заболевших коронавирусной инфекцией было 3 (17,6%) пациента с ожирением, 3 (17,6%) – с гипертонической болезнью, 2 (11,8%) пациента имели длительный стаж курения – более 10 пачек/лет. Кроме того, пациенты (27 человек), включенные в наше исследование, в рамках проекта массовой вакцинации населения РФ против новой КВИ были обследованы на уровень антител класса G к COVID-19. У 11 (15,3%) пациентов обоих групп перед планируемым введением вакцины «Спутник-V» выявлены антитела класса G, что свидетельствовало о наличии у них бессимптомного течения болезни. Вероятно, отсутствие тяжелых случаев COVID-19, обострений БА на фоне инфекции связано с несколькими факторами, а именно с достаточно молодым возрастом пациентов, хорошим контролем астмы, обеспеченным базисными препаратами и СЛИТ. Возможно, постоянное применение ИГКС подавляет воспалительный ответ со стороны слизистой оболочки верхних и нижних дыхательных путей, вызванный воздействием вируса. Кроме того, ИГКС снижают уровень провоспалительных цитокинов и одновременно повышают уровень противовоспалительных цитокинов, что может иметь положительное значение [26]. Проведение АСИТ, действие которой направлено на регуляторные Т-клетки, также способствует подавлению воспалительной реакции и препятствует активации и пролиферации остальных подтипов Т-клеток, что улучшает прогноз при COVID-19. Таким образом, АСИТ и регулярная базисная терапия в соответствии со степенью тяжести больных БА приводят к улучшению контроля заболевания, что немаловажно и для выздоровления в случае COVID-19.
Заключение
СЛИТ у пациентов с аллергической БА способствует уменьшению частоты и выраженности симптомов заболевания, частоты обострений, повышению контроля и снижению объема базисной терапии. Клинико-иммунологическая эффективность СЛИТ выражается в положительной динамике ряда иммунологических параметров. СЛИТ характеризуется благоприятным профилем безопасности и удобства в связи с тем, что возможно применение ее вне медицинского учреждения, осуществление наблюдения за пациентами допустимо с использованием телемедицинских технологий. На фоне СЛИТ наблюдалось более благоприятное течение сопутствовавших заболеваний, что, вероятно, отражает ее системный лечебный эффект.
Для безопасного проведения СЛИТ в условиях пандемии COVID-19 следует верифицировать инфицирование COVID-19, поскольку инфекция считается временным противопоказанием к ее проведению. Пациенты с контролируемой БА не подвержены большему риску заражения и тяжелого течения COVID-19. Базисная терапия БА позволяет быстрее добиваться и поддерживать контроль БА, что значительно облегчает проведение иммунотерапии. Лучший контроль БА обеспечивается комбинацией базисной терапии и СЛИТ. Применение комбинации базисного лечения и СЛИТ в условиях пандемии COVID-19 для лечения больных БА позволяет не только обеспечивать лучший контроль над заболеванием, но и минимизировать риски обострений, тем самым снизить частоту обращения за помощью, оказание которой в настоящих условиях довольно затруднительно.
Таким образом, АСИТ может быть эффективной и безопасной даже в обстановке пандемии КВИ при условии достижения и поддержания оптимального фармакологического контроля болезни, а также, возможно, данный метод лечения обеспечивает лучшие прогнозы в отношении инфекции COVID-19 у пациентов с БА вследствие повышения ее контроля.



