Введение
Лечение больных метастатической меланомой остается непростой задачей, несмотря на впечатляющие достижения лекарственной терапии. Результаты недавно проведенных клинических исследований демонстрируют почти двукратное увеличение общей выживаемости (ОВ) и выживаемости без прогрессирования (ВБП) при использовании как лекарственных препаратов, действующих на опухоли с мутацией в гене BRAF [1–4], так и модуляторов иммунологического синапса, в т.ч. больными без мутации в гене BRAF [5–8].
Эффективность терапии, направленной против опухоли с мутацией в гене BRAF, по данным клинических исследований, оказывается весьма высокой в первой линии лечения и несколько снижается при использовании этих лекарственных средств во второй и последующих линиях терапии [2].
Однако в реальной клинической практике в РФ пациенты, как правило, не получают наиболее эффективного лечения в первой линии терапии. В данной работе мы провели анализ эффективности и переносимости терапии комбинацией ингибитора BRAF, дабрафениба, и ингибитора МЕК, траметиниба, пациентами с метастатической меланомой, принимавшими участие в Программе расширенного доступа к препаратам в отделении биотерапии опухолей РОНЦ им. Н.Н. Блохина Минздрава России.
Пациенты и методы
Критерии включения и невключения в программу
Основные критерии для участия пациентов в программе: возраст старше 18 лет, добровольное информированное согласие, наличие мутации в гене BRAF (независимо от ее типа), способность принимать пероральные лекарственные препараты, общее состояние по шкале ECOG от 0 до 3, стабильное клиническое состояние, адекватная контрацепция для женщин детородного возраста.
Основные критерии невключения пациентов в программу: беременность или кормление грудью, необходимость проведения другого противоопухолевого лечения, наличие другой (второй) злокачественной опухоли, выявленной в течение года или позднее до планируемого включения в программу, в которой возможна активация сигнального пути RAS (специального тестирования на мутацию в гене RAS проводить не требовалось).
Следует отметить, что к участию в программе допускали пациентов, ранее получавших ингибиторы BRAF или МЕК (кроме дабрафениба и траметиниба) и не имевших признаков прогрессирования на фоне их приема (т.е. предшествовавшее лечение ингибиторами BRAF или МЕК могло быть прекращено по причине непереносимости, отсутствия доступа к препарату или случаев прогрессирования, когда новые очаги поражают исключительно ЦНС).
К участию в программе не допускали лиц с известной аллергией на компоненты препарата (например, ДМСО), высоким риском окклюзии вен сетчатки, острым коронарным синдромом или застойной сердечной недостаточностью 3-го и 4-го функциональных классов по классификации Нью-Йоркской ассоциации сердца (NYHA).
Исследуемый препарат и ход лечения
Препараты для Программы расширенного доступа были любезно предоставлены компанией ГлаксоСмитКляйн, далее по ходу продолжения программы дополнительные поставки препаратов осуществлены компанией Новаритс, к которой в тот период времени перешли права на регистрационные свидетельства дабрафениба и траметиниба.
Пациентам, удовлетворявшим критериям включения и не имевшим критериев исключения, выдавали препарат дабрафениб, который следовало принимать в дозе 150 мг (две капсулы по 75 мг) внутрь 2 раза в сутки, и траметиниб, который следовало принимать по 1 таблетке (2 мг) 1 раз в сутки.
Лечение продолжалось до непереносимости терапии, не купируемой снижением дозы, или до прогрессирования заболевания. При этом допускалось продолжение терапии после наступления прогрессирования в случае, если врач-исследователь видел в этом пользу.
Оценку эффекта терапии проводили каждые 2–3 месяца. Для оценки эффекта использованы критерии RECIST 1.1 [9].
Статистический анализ
Перед началом программы никакого статистического анализа не было запланировано. Для описания характеристик пациентов были использованы методы описательной статистики, для оценки ОВ и ВБП – метод Каплана–Мейера. Статистические расчеты выполнены в программе IBM SPSS Statistics Professional 20.0.
Результаты
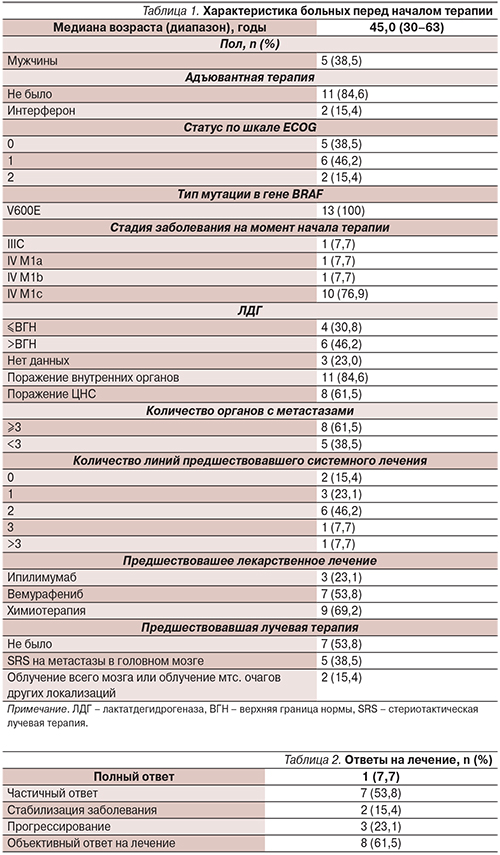
В период с марта 2015 по июнь 2015 г. в Программу расширенного доступа к препаратам в России были включены 14 больных. Сведения об 1 пациенте (который получал лечение в течение менее 2 месяцев) на момент написания данной статьи были недоступными и исключены из анализа. Основные характеристики пациентов (n=13) приведены в табл.1.
На момент подготовки данной работы медиана периода наблюдения составила 20,1 месяца, прогрессирование было зафиксировано у 10 (77%), из 13 пациентов умерли 7 (54%) из 13 больных.
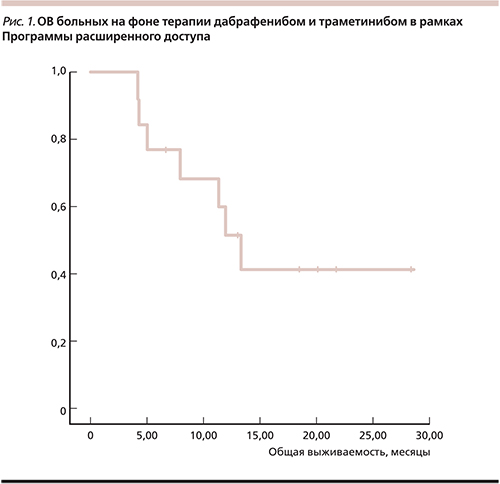
Медиана ОВ составила 13,27 месяца (95% доверительный интервал [ДИ] – 10,3–16,2), одногодичная ОВ – 53%, двугодичная – 43% (рис. 1). С учетом малого числа пациентов подгрупповой анализ не выполнялся.

Медиана ВБП составила 7,29 месяца (95% ДИ – 3,74–10,84), одногодичная ВБП – 31%, двугодичная – 22% (рис. 2).
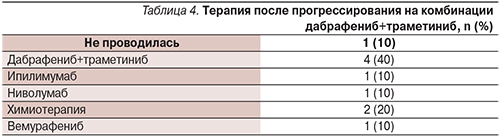
Частота объективных ответов на лечение составила 61,5% (8 из 13 больных), медиана длительности ответа на лечение – 7,72 месяца (95% ДИ – 2,03–13,41; табл. 2), медиана времени до регистрации объективного ответа на лечение – 2 месяца (95% ДИ – 1,00–3,78).
Мы также проанализировали характеристики пациентов с различным типом ответа на лечение (табл. 3). Выполненные статистические сравнения (критерий Хи-квадрат) не выявили различий между подгруппами пациентов, что, вероятно, связано с малым числом больных в подгруппах. Динамика индивидуальных размеров таргетных опухолевых узлов (в соответствии с RECIST 1.1) представлена на рис. 3.
Мы также проанализировали возможные различия в отношении ряда клинических и лабораторных признаков у пациентов с длительным (>12 месяцев) и коротким периодом без прогрессирования (<12 месяцев). При статистическом анализе (критерий Хи-квадрат) были выявлены статистические различия в отношении распространенности заболевания (распространенность, соответствующая стадии M1c, оказывала достоверно более негативное влияние на прогноз по сравнению с меньшей распространенностью болезни) и общим состоянием по шкале ECOG (лучшее состояние соответствовало большей ВБП) (табл. 3).
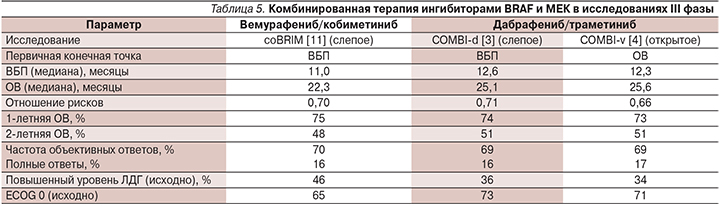
Медиана длительности лечения составила 8,08 месяца (диапазон – 1,00–28,29). В связи с окончанием Программы расширенного доступа пациенты, у которых не было зафиксировано прогрессирования заболевания, были переведены на другие варианты терапии: 1 больная не получает лечения в течение 13 месяцев после достижения полного эффекта, 1 пациентка получает вемурафениб (не получала его ранее), 1 больная продолжает получать комбинацию дабрафениба и траметиниба. У одного пациента прогрессирование наступило спустя 2 месяца после переключения на монотерапию дабрафенибом. Варианты терапии после прогрессирования на комбинированном лечении дабрафенибом и траметинибом суммированы в табл. 4. Следует отметить, что четверым больным терапия дабрафенибом и траметинибом была продолжена после регистрации прогрессирования по критериям RECIST и в среднем продолжалась еще 4,3 месяца до наступления клинического ухудшения состояния, поскольку другие лечебные опции были недоступными. В большинстве случаев прогрессирование проявлялось в виде появления новых опухолевых узлов (в т.ч. в головном мозге) при дальнейшей тенденции к сокращению описываемых ранее опухолевых узлов. В случаях когда позволяла локализация, новые узлы подвергались хирургическому удалению, а лекарственная терапия продолжалась в прежнем объеме. Однако повторное радиологическое исследование через 2–4 месяца демонстрировало появление новых дополнительных опухолевых очагов и в ряде случаев увеличение ранее существовавших.

Со стороны пациентки, получившей ипилимумаб после прогрессирования на дабрафенибе и траметинибе, наблюдался частичный эффект, при этом не было отмечено сколько-нибудь выраженных нежелательных явлений. У пациента, получившего ниволумаб после прогрессирования на фоне приема дабрафениба и траметиниба, равно как и у больных, получивших вемурафениб и химиотерапию, отмечено прогрессирование заболевания.
Лечение комбинацией дабрафениба и траметиниба не сопровождалось выраженными нежелательными явлениями. У двух пациентов была отмечена пирексия 2-й степени тяжести по СТСАЕ v 4.03 (подъемы температуры тела до 39–40°С), от 1 из них это нежелательное явление потребовало перерыва в приеме препарата и редукции дозы на одну ступень. Кожная сыпь, гиперкератоз по выраженности не превышали 1-й степени тяжести по СТСАЕ. Лабораторные отклонения (главным образом анемия, нейтропения, гиперферментемия АЛТ и АСТ) также не превышали 1-й степени тяжести. За время наблюдения не было отмечено серьезных нежелательных явлений, связанных с применением препаратов.
Обсуждение
Комбинация ингибиторов BRAF и МЕК представляет собой хорошо переносимый и высокоэффективный вариант системной терапии больных метастатической или нерезектабельной меланомой кожи IIIC- и IV-стадий с мутацией в гене BRAF.
Данные крупных международных рандомизированных исследований демонстрируют впечатляющие результаты, которые не всегда удается воспроизвести в условиях реальной клинической практики. Так, например, в исследованиях III фазы по изучению комбинированной терапии ингибиторами BRAF и МЕК (табл. 5) показатели медианы ОВ превышали 2 года, в то время как при анализе данных, полученных в Программах расширенного доступа к препарату (намного более приближенных к условиям реальной клинической практики) результаты лечения уже несколько хуже: частота объективных ответов (без анамнеза терапии ингибиторами BRAF) составила 61,9% (полные ответы – 10,8%; частичные ответы – 51,1%), а частота стабилизации заболевания – 9,4%. Медиана ВБП составила 7,4 месяца, медиана ОВ – 20 месяцев [10]. В нашей работе мы получили очень близкие данные, что подтверждает хорошую вопроизводимость результатов терапии.
Небольшое число пациентов, принимавших участие в Программе расширенного доступа, не позволяет выполнить сколько-нибудь значимый статистический анализ факторов успеха или неудачи терапии. Единственными характеристиками, оказавшими влияние на длительность периода без прогрессирования, были распространенность болезни, оцененная по системе TNM как соответствующая M1c (поражение внутренних органов и/или повышенный уровень ЛДГ) и состояние по шкале ECOG. Однако результаты сравнения подгрупп пациентов с различными исходами лечения в целом согласуются с данными, полученными в крупных рандомизированных исследованиях, свидетельствующих, что меньший объем болезни, хорошее функциональное состояние по шкале ECOG, низкий уровень ЛДГ служат предиктором успеха терапии [12]. Это означает, что пациенты с набором «благоприятных» признаков (как правило, это бывает в дебюте метастатической болезни) могут получать наибольшую пользу от комбинированной терапии ингибиторами BRAF в комбинации с ингибиторами MEK, и нет никаких убедительных научных оснований откладывать начало такой терапии на вторую и последующие линии. С другой стороны, у пациента с набором всех неблагоприятных признаков (высокий уровень ЛДГ, плохой ECOG-статус, большая распространенность заболевания) также имеются все основания для начала эффективной терапии. Прирост выживаемости в случае наличия неблагоприятных признаков в абсолютном выражении будет безусловно ниже, но даже в этих условиях комбинация ингибиторов BRAF+MEK оказывает положительное действие и может иметь преимущество по сравнению с химио- или монотерапией ингибиторами BRAF.
Следует также отметить, что в нашей серии наблюдений часть пациентов ранее уже получала вемурафениб и перешла на комбинацию дабрафениба и траметиниба в связи с отсутствием доступа к препарату или прогрессированием (появление новых очагов в ЦНС). Важно отметить, что все больные, у которых была отмечена первичная резистентность к комбинации (3 из 13), ранее получали вемурафениб, однако прием этого препарата не воспрепятствовал объективному ответу на лечение от 3 из 8 пациентов, у которых такой ответ был зарегистрирован.
При наступлении прогрессирования на фоне применения комбинации ингибиторов BRAF и МЕК часть пациентов продолжила терапию по прежней схеме. В нашей серии наблюдений для большинства пациентов не было каких-либо других доступных лечебных опций, за исключением продолжения терапии. Во всех случаях явления прогрессирования были отмечены и при последующих радиологических исследованиях. Опыт международных исследований также говорит о том, что продолжение прежней терапии не приводит к сколько-нибудь значимому увеличению частоты объективного ответа и может приводить лишь к непродолжительной стабилизации [13, 14]. В нашей серии наблюдений применение модуляторов иммунологического синапса после прогрессирования заболевания на фоне приема ингибиторов BRAF и МЕК было весьма ограниченным, тем не менее 1 случай частичного ответа на ипилимумаб не может не вселять оптимизма.
Переносимость комбинированной терапии ингибиторами BRAF и МЕК оказалась хорошей. Не было отмечено ни одного серьезного нежелательного явления. Выраженность кожной токсичности (сыпь, зуд, гиперкератоз) не превышала 1–2-й степеней тяжести, и они не были потенцированы приемом вемурафениба в анамнезе. У одного пациента была отмечена пирексия, не купируемая НПВС и стероидами, что потребовало приостановки приема препаратов на 3 дня с последующей редукцией дозы на 1 ступень. Отклонения в лабораторных показателях также были весьма умеренными и никогда не требовали приостановки лечения или коррекции дозы.
Заключение
Комбинация ингибиторов BRAF и МЕК дабрафениба и траметиниба представляет собой высокоэффективный и хорошо переносимый вариант лечения метастатической или нерезектабельной меланомы с мутацией в гене BRAF V600.
Эффективность и безопасность данного варианта лечения были доказаны в рамках крупных многоцентровых исследований. В нашей работе приведены результаты применения комбинации дабрафениба и траметиниба в условиях реальной клинической практики в российской популяции пациентов. Полученные данные хорошо согласуются с ранее опубликованными результатами рандомизированных исследований. Большая часть больных получает пользу от применения указанных препаратов как в первой, так и в последующих линиях лечения. Поиски причин первичной и приобретенной резистентности к такой терапии и способы их преодоления требуют дополнительных исследований.
Благодарности
Авторский коллектив благодарит сотрудников компании GSK и Novartis Александра Гика, Евгения Михайлова и Ольгу Матвееву за усилия, приложенные для реализации Программы расширенного доступа к выскоэффективному лечению в России. Мы также выражаем благодарность сотрудникам отделения биотерапии опухолей, принимавших участие в курации пациентов, самих пациентов и их семей за терпение и сотрудничество в процессе лечения.



