Введение
Артериальная гипертензия (АГ) – ведущий фактор сердечно-сосудистой заболеваемости и смертности, затрагивающий около 30% взрослого населения [1]. На долю эссенциальной АГ приходится 70–80% всех случаев АГ. Среди вторичной АГ наиболее частой причиной является первичный гиперальдостеронизм (ПГА), встречающийся в 5–10% случаев [2]. При данном заболевании риск развития сердечно-сосудистых событий и метаболических нарушений значительно выше, чем у пациентов с эссенциальной АГ, даже при достижении целевых значений артериального давления (АД). С учетом низкой осведомленности врачей первичного звена о важности своевременной диагностики ПГА, существующих сложностей в диагностике мягких форм данного состояния проблема низкой выявляемости автономной гиперпродукции альдостерона является крайне актуальной во всем мире [3].
Новое в патогенезе
Ключевым моментом патогенеза ПГА является автономная продукция альдостерона, которая может быть представлена односторонним 40%-ным и двусторонним 60%-ным поражением надпочечников, подтвержденным данными селективного забора крови из надпочечниковых вен (СВЗК). Клинически ПГА проявляется АГ, у 30% пациентов развивается спонтанная или возникшая на фоне приема диуретиков гипокалиемия, в таком же проценте случаев имеет место синдром обструктивного апноэ сна. В пользу ПГА могут свидетельствовать тенденция к высоконормальным значениям натрия, жалобы на учащенное ночное мочеиспускание, наличие нарушений углеводного обмена и депрессии [3–5].
Ранее считалось, что АГ является облигатным признаком ПГА. Проведенные в последние годы исследования изменили это представление. В одном из них J.M. Brown et al. продемонстрировали, что автономная гиперпродукция альдостерона встречается и среди пациентов без АГ, при этом нормальные значения АД, по-видимому, являются результатом эффективной работы компенсаторных механизмов. Можно говорить о наличии положительной корреляции степени гиперпродукции альдостерона со степенью повышения АД. В исследуемой группе у пациентов без АГ ПГА был подтвержден в 11,3% случаев, при АГ 1-й степени – в 15,7%, АГ 2-й – в 21,6% и АГ 3-й степени – в 22% [6].
Гистологически двусторонняя гиперпродукция альдостерона может быть представлена микро-, макронодулярной либо диффузной гиперплазией, редко – двусторонней альдостерон-продуцирующей аденомой (АПА). Односторонняя гиперпродукция может быть представлена АПА, различными вышеперечисленными формами гиперплазии либо сочетанием аденомы с гиперплазией. Для каждого из перечисленных фенотипов ПГА характерны определенные соматические мутации ионных каналов.
В настоящее время известны мутации в генах калиевых каналов-5 (KCNJ5), вольтаж-зависимых кальциевых каналов (CACNA1D), Na+/K+ АТФ-азы (ATP1A1) и Ca2+ АТФ-азы-3 (ATP2B3). Перечисленные мутации прямо или косвенно повышают внутриклеточную концентрацию кальция, увеличивая экспрессию и активность фермента клубочковой зоны коры надпочечников – альдостеронсинтазы CYP11B2 [7, 8]. Мутация KCNJ5 коррелирует с более выраженным поражением сердечно-сосудистой системы, в частности гипертрофией левого желудочка, которая может регрессировать после оперативного лечения [9]. Примечательно, что даже в надпочечниках здоровых людей обнаруживают участки микронодулярной гиперплазии с автономной продукцией альдостерона неопухолевой природы. Их можно идентифицировать с помощью окрашивания гематоксилином и эозином, а также иммуногистохимическим (ИМГХ) исследованием антител к альдостеронсинтазе (CYP11B2).
Описаны кластеры альдостерон-продуцирующих клеток, диффузно расположенных в надпочечнике, в клубочковой и пучковой зонах, а также непосредственно под капсулой надпочечника. Их количество увеличивается с возрастом. Предполагают, что данные кластеры являются предшественниками альдостером, т.к. обе нозологии ассоциированы с одними мутациями (в генах CACNA1D, ATP1A1 и ATP2B3).
Диагностика
Успешность лечения ПГА во многом определяется своевременной диагностикой до развития необратимых осложнений. ПГА ассоциирован с более высоким риском развития неблагоприятных сердечно-сосудистых событий и метаболических нарушений, чем у пациентов с эссенциальной АГ [10–12] даже при достижении целевых значений АД. В частности, доказан повышенный риск развития фибрилляции предсердий, инсульта (отношение шансов [ОШ]=2,58), ишемической болезни сердца (ОШ=1,77), гипертрофии левого желудочка (OR=2,29), сердечной недостаточности (ОШ=2,05), сахарного диабета (ОШ=1,33) и метаболического синдрома (ОШ=1,53) [4].
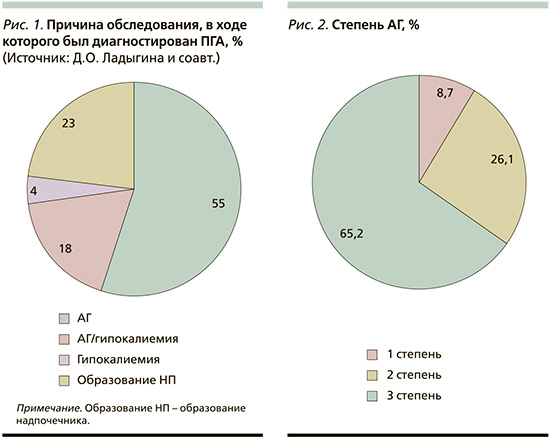
Проведен ретроспективный анализ данных 43 пациентов, поступивших в УКБ № 2 ПМГМУ им. И.М. Сеченова за период с ноября 2022 по февраль 2024 г., прооперированных ранее по поводу ПГА. Медиана возраста на момент оперативного вмешательства составила 46 лет [37; 58], со средней длительностью АГ до диагностики ПГА – 10 лет (8–17). Причиной обследования, в ходе которого был диагностирован ПГА, в 55% случаев являлось наличие АГ, в 18% – сочетание АГ с гипокалиемией. В 23% первичным поводом для исключения ПГА было обнаружение инциденталомы надпочечников (рис. 1). На момент операции 66,2% пациентов имели АГ 3-й степени (рис. 2). У подавляющего большинства пациентов (35 из 43) в анамнезе зарегистрирована гипокалиемия с медианой 2,75 ммоль/л (2,53–3,08). Гипертрофия ЛЖ при предоперационном обследовании выявлена у 50% пациентов, при этом фибрилляция предсердий, острый инфаркт миокарда, острое нарушение мозгового кровообращения в анамнезе отмечены у 4,5%. Анализ функционального состояния почек на дооперационном этапе показал, что медиана скорости клубочковой фильтрации (СКФ) составила 100 мл/мин/1,73 м2 (81–108), при этом при распределении пациентов по уровню СКФ хроническая болезнь почек стадий С1–2 была у 76,2% участников, С3а – у 14,3%, С3б – у 4,8% и С4 – у 4,8% пациентов.
Несмотря на очевидно негативные последствия воздействия хронического избытка альдостерона на органы-мишени, частота скрининга ПГА остается низкой. Причина заключается в отсутствии единого консенсуса, простого алгоритма диагностики и лечения данного состояния. Помимо этого, по мнению авторов этой статьи, не во всех клинических рекомендациях кардиологических сообществ сделан акцент на важности своевременной диагностики ПГА [13–15]. В рекомендациях Европейского общества кардиологов по лечению артериальной гипертензии – ESH (European Society of Hypertension) скрининг ПГА обсуждается лишь в контексте пациентов с резистентной АГ (приблизительно 10% всех пациентов) [3, 16, 17]. В руководстве Национального института здравоохранения и совершенствования медицинской помощи Великобритании о необходимости скрининга ПГА не упоминается вовсе [3, 18, 19].
Рекомендации эндокринологического сообщества по диагностике и лечению ПГА (Endocrine Society guidelines, 2016) выделяют следующие группы риска для проведения скрининга: пациенты с устойчивым АД >150/100 мм рт.ст. при каждом из трех измерений, полученных в разные дни; с АГ (АД>140/90 мм рт.ст.), резистентной к трем стандартным антигипертензивным препаратам (включая диуретик) или контролируемым АД (<140/90 мм рт.ст.) при приеме четырех и более антигипертензивных препаратов; пациенты с АГ в сочетании со спонтанной или индуцированной диуретиками гипокалиемией; пациенты с АГ и инциденталомой надпочечников; пациенты с АГ и апноэ сна; пациенты с АГ и семейным анамнезом ранней АГ или нарушениями мозгового кровообращения в молодом возрасте (<40 лет); при наличии АГ у родственников первой степени родства пациентов с ПГА [20].
При анализе реальной частоты исключения ПГА в рутинной клинической практике получены удручающие результаты. В ходе популяционного ретроспективного когортного исследования, проведенного в Канаде, установлено, что исключение ПГА было проведено только 3,9% пациентов с гипокалиемией <3,0 ммоль/л и повышенным АД, а также 1% пациентов с резистентной АГ [21]. При наличии резистентной АГ, по данным Стэнфордской системы здравоохранения, обследование на ПГА проведено в 2,1% случаев 4660 пациентам [22], по данным Университета Миннесоты – в 4,2% случаев 18 908 пациентам [23]. Аналогичные результаты получены в Чикагском университете, где выявление гипокалиемии в сочетании с АГ послужило поводом для дальнейшего обследования только в 2,7% случаев в когорте 36 941 пациента [24].
Низкая информированность о ПГА и как следствие – поздняя диагностика задерживают назначение патогенетически оправданной терапии или проведение оперативного лечения, в связи с чем большинство случаев ПГА выявляется после развития необратимых осложнений [25]. Повышенное АД и патологическое воздействие альдостерона на органы-мишени [3, 26–28] приводят к эндотелиальной дисфункции, воспалению, фиброзу [29, 30], что значительно уменьшает шансы на излечение от АГ после хирургического лечения [31–34]. В связи с этим ряд авторов настаивают на необходимости скрининга на ПГА всех пациентов с впервые выявленной АГ до назначения терапии вне зависимости от других сопутствующих факторов. Такой подход позволил бы значительно нивелировать проблему оценки влияния принимаемых антигипертензивных препаратов на уровень ренина и альдостерона, необходимость коррекции терапии, отмены определенных групп препаратов перед лабораторной диагностикой. Инертность врачей, крайне низкая частота назначения анализов для исключения ПГА во многом связаны с многоэтапностью диагностики, отсутствием простых, понятных врачам первичного звена алгоритмов первичной диагностики.
В интерпретации результатов скрининга также не приходится говорить о единстве подходов среди как российских эндокринологов, так и зарубежных коллег. Несмотря на описанные в клинических рекомендациях отрезные точки по показателям альдостерона, ренина и альдостерон-ренинового соотношения (АРС), выход за пределы которых является основанием для проведения подтверждающих тестов, большинство врачей по-прежнему, получая результат альдостерона в верхней трети референса в сочетании с задавленным ренином, не воспринимают это как показание к проведению дальнейших диагностических этапов. Другая распространенная ошибка заключается в попытке интерпретировать результат только по уровню альдостерона без исследования показателей ренина.
В настоящее время методом первичного исключения ПГА является определение концентрации альдостерона и прямого ренина плазмы (либо активности ренина плазмы – АРП). При наличии сомнений в интерпретации полученных результатов проводится расчет АРС. Оптимальными условиями забора крови являются утренние часы до 10.00, примерно через 2 часа после пробуждения, при этом по возможности рекомендуется на протяжении этого времени находиться в вертикальном положении (сидя/стоя). Положительным в отношении ПГА считается уровень альдостерона плазмы >277 пмоль/л (10 нг/дл; 100 пг/мл) и АРП <1,0 нг/мл/ч или концентрация ренина ниже референсного диапазона [20, 35]. Такие значения альдостерона и ренина по большому счету уже не требуют расчета АРС.
В последние годы активно обсуждается вопрос субклинических и «мягких» форм гиперальдостеронизма, при которых сохраняются нормальные показатели АД (11,3%) или присутствует легкая (15,7%) или умеренная АГ (21,6%) [6]. Использование более высоких значений уровня альдостерона, чем упомянутые выше, будет препятствовать выявлению этих форм ПГА. Важно помнить, что даже в группе пациентов с подтвержденным ПГА при 3-кратном динамическом контроле альдостерона у 30% пациентов одно из значений было менее 10 нг/дл. Не задавленные, а низконормальные значения ренина также могут наблюдаться при «мягких» формах ПГА, определение АРС в этих случаях может помочь определиться с дальнейшей тактикой [29].
Эффективность применения патогенетически оправданной терапии антагонистами минералокортикоидных рецепторов (АМКР) при «мягких» формах ПГА была ярко продемонстрирована в исследовании PATHWAY-2. Применение АМКР пациентами с уровнем альдостерона в верхней трети референса в сочетании с задавленным ренином позволило достигать целевых значений АД. Данные результаты подчеркивают, что ключевым параметром в диагностике ПГА является подавленный уровень ренина даже в отсутствие выраженного повышения уровня альдостерона [36, 37].
J. Funder предлагает использовать в качестве первичного теста для исключения ПГА оценку суточной экскреции альдостерона с мочой до назначения антигипертензивной терапии. ПГА исключается, если экскреция альдостерона с мочой <6 мкг, подтверждается, если экскреция альдостерона с мочой >12 мкг при условии уровня экскреции натрия с мочой >200 ммоль на фоне высокосолевой диеты. При экскреции альдостерона с мочой в диапазоне от 6 до 12 мкг, т.н. серая зона, ПГА вероятен, при этом рекомендуется пробный прием спиронолактона 25 мг/сут в течение 4 недель с динамической оценкой уровня АД. При снижении АД менее чем на 10 мм рт.ст., ПГА маловероятен. Если уровень снижения АД>10 мм рт.ст., ПГА высоковероятен, причем чем выше падение, тем больше его вероятность [20].
Как правило, положительный результат скрининга подтверждают одним из функциональных тестов: подавляющий тест с флудрокортизоном, пероральный нагрузочный тест с натриевой нагрузкой, тест с инфузией физиологического раствора или тест с каптоприлом. Подтверждающий тест может проводиться на фоне терапии, которая была изначально, если на ней достигнуты обозначенные выше критерии диагностики. Исключением, когда подтверждающих тестов не требуется, являются случаи манифестного гиперальдостеронизма со спонтанной гипокалиемией, задавленной ренином плазмы и уровнем альдостерона >20 нг/дл (550 пмоль/л). Последний, для простоты запоминания, в 2 и более раза должен превышать обозначенную отрезную точку для первого этапа диагностики.
Топическая диагностика
Дифференциальный диагноз одно- и двусторонней автономной продукции альдостерона, которая, как мы упоминали выше, может быть гистологически представлена различными вариантами одно- либо двусторонней гиперплазии с возможным сочетанием с АПА, имеет определяющее значение для выбора дальнейшей тактики лечения. Диагностика фенотипа ПГА основана на компьютерной томографии (КТ) в сочетании с СВЗК. В России проведение СВЗК возможно только по протоколу нестимулированного козитропином забора крови [35].
При визуализации надпочечников, по данным КТ, при ПГА можно увидеть неизмененные либо диффузно-увеличенные надпочечники, одно/двусторонние микро- (<1 см) или макроаденомы. На КТ АПА представляет собой образование небольшого размера 1–3 см в диаметре низкой нативной плотности (<10 единиц Хаунсфилда – HU), что соответствует доброкачественному фенотипу. Альдостерон-продуцирующая карцинома встречается крайне редко, как правило, характеризуется выраженными биохимическими отклонениями (уровень калия в сыворотке крови <2,5 ммоль/л), тяжелой АГ, высокой нативной плотностью образования надпочечников (>20 HU) >4 см в диаметре. Необходимо учитывать, что КТ не позволяет оценивать функциональную активность образования надпочечника, в связи с чем гормонально-неактивные образования, частота наличия которых увеличивается с возрастом, нередко ошибочно интерпретируют как АПА [38]. СВЗК может не проводиться в двух случаях: если пациент моложе 35 лет, значения альдостерона трижды превышают выше обозначенную отрезную точку в сочетании с гипокалиемией, задавленным уровнем ренина и наличием образования в одном из надпочечников, соответствующего по характеристикам АПА. По результатам нашего наблюдения за 43 пациентами описаны данные характеристики каждого третьего (33%) участника. Также проведение СВЗК не показано при наличии доказанной автономной продукции кортизола, которая была выявлена у 12,5% пациентов. Сочетание обеих причин наблюдалось в 4,3% случаев, и в 4,3% случаев результаты СВЗК были не информативными, тем не менее принято решение о проведении односторонней адреналэктомии по причине тяжелого течения ПГА. Большинство АПА представляет собой один четко очерченный узел, при этом довольно часто в удаленной железе обнаруживают макро- или микронодулярную гиперплазию [39] с различной степенью ремоделирования коры надпочечников [40].
Инвазивный характер и малая доступность СВЗК резко ограничивают возможность проведения диагностики для уточнения источника гиперпродукции альдостерона и выбора оптимальной тактики лечения. В связи с этим в настоящее время продолжается поиск альтернативных методов латерализации, один из них – ПЭТ (позитронно-эмиссионная томография) -КТ с 11С-метомидатом. Метомидат – производное метилового эфира на основе имидазола.
Благодаря высокой афинности он связывается со стероидогенными ферментами надпочечников – 11β-гидроксилазой (CYP11B1) и альдостеронсинтазой (CYP11B2), которые контролируют заключительные этапы синтеза глюкокортикоидов и минералокортикоидов соответственно Радиоактивная метка 11C при распаде высвобождает позитрон, который выступает в качестве радиоиндикатора ПЭТ и накапливается в зонах гиперфункции коры надпочечников [3, 41]. С целью повышения чувствительности при определении латерализации одностороннего ПГА необходимо за 72 часа до исследования начать прием дексаметазона, чтобы подавить экспрессию CYP11B1 и выявить CYP11B2-положительные участки функциональной автономии. Результаты проспективного исследования подтверждают, что точность СЗКВ и ПЭТ-КТ с 11С-метомидатом сопоставимы и ПЭТ-КТ является надежным неинвазивным аналогом для диагностики варианта ПГ [42]. Важным ограничением ПЭТ-КТ с 11С-метомидатом является короткий период полураспада (T1/2 20 минут) радиофармпрепарата, поэтому сканирование доступно только в местах, где имеется циклотрон. В настоящее время ведется разработка более стабильных изотопов, таких как 18F-CETO (пара-хлор-2-(18F)-фторэтилетомидат), с более длительным периодом полураспада (T1/2 110 минут), что позволит в будущем увеличить доступность визуализации с помощью молекулярных изотопов [3].
Иммуногистохимия
Одним из методов оценки источника функциональной активности и гистопатологической классификации ПГА является иммуногистохимическое (ИГХ) исследование с использованием антител к альдостеронсинтазе (CYP11B2). Альдостеронсинтаза – специфический фермент, представленный только в клетках клубочковой зоны надпочечников, контролирующий превращение дезоксикортикостерона в альдостерон на заключительном этапе стероидогенеза [8].
На основе оценки иммунореактивности CYP11B2 выделено два гистологических подтипа ПГА – АПА и микронодуллярная гиперплазия, каждый из которых может быть представлен как односторонней, так и двусторонней локализацией. Причем от 13 до 30% случаев составляют микроаденомы, которые не визуализируются на КТ, но подтверждены результатами СВЗК и ИГХ-исследованиями [43]. Как правило, для аденом характерна изолированная экспрессия CYP11B2, в более редких случаях встречается сочетанная экспрессия CYP11B2, CYP11B1 и 17α-гидроксилазы [8, 44]. Это объясняет причину положительного ночного подавляющего теста с дексаметазоном у ряда пациентов с ПГА и более тяжелый прогноз сердечно-сосудистых и метаболических нарушений [4, 45].
Экспрессия CYP11B2 отмечена не только в клубочковой зоне, но и в специфических морфологических структурах, т.н. кластерах клеток, продуцирующих альдостерон (APCC – aldosterone produced cell clasters). Кроме CYP11B2, в АРСС также зарегистрирована экспрессия 3b-HSD2, при этом отсутствуют маркеры клеток клубочковой и пучковой зон, в связи с чем можно предположить, что АРСС – промежуточная зона между клубочковой и сетчатой зонами. APCC обнаруживается как в нормальной коре надпочечников, так и в прилежащих к АПА участках, автономно продуцирующих альдостерон [46].
Наборы для секвенирования ДНК нового поколения совместно с ИГХ-исследованием антител к CYP11B2 позволили идентифицировать мутации ионных каналов CACNA1D, ATP1A1 и ATP2B3 в APCC нормальных надпочечников. Плотность расположения APCC значительно выше в надпочечниках с двусторонней гиперплазией, чем у пациентов контрольной группы с нормотензией. Это послужило основой для предположения, что APCC является предшественником АПА. Примечательно, что данная гипотеза подтверждается наличием специфических трансляционных повреждений (pAATL: APCC-to-APA), которые включают морфологические признаки, характерные как для АРСС, так и для АПА [47]. Мутации генов-драйверов APA были идентифицированы в разных частях pAATL; в одном случае соматическая мутация KCNJ5 идентифицирована только в mAPA-подобной структуре, тогда как в другом случае соматическая мутация ATP1A1 была идентифицирована во всей структуре. Требуется проведение дальнейших исследований для определения стадийности процесса формирования АПА.
Лечение
Тактика лечения ПГА определяется в зависимости от фенотипа, продолжительности и тяжести течения ПГА, наличия сопутствующих заболеваний. При согласии пациента и отсутствии абсолютных противопоказаний к операции в случае доказанной односторонней гиперпродукции альдостерона оптимальным выбором является хирургическое лечение, в отсутствие латерализации по данным СВЗК или невозможности его проведения предпочтение отдается медикаментозной терапии. Преимуществом хирургического лечения является полное удаление источника гиперпродукции альдостерона, которая приводит к снижению смертности на 66% и риска основных неблагоприятных сердечно-сосудистых событий (MACE – major adverse cardiovascular outcomes) на 45% по сравнению с медикаментозной терапией. Снижение смертности при этом происходит как от всех причин, так и по причине неблагоприятных сердечно-сосудистых событий. По результатам наблюдения за 200 прооперированными пациентами в течение 12 месяцев приходилась одна предотвращенная смерть по сравнению с медикаментозной терапией. Хирургическое лечение способствует нормализации АД у 30–60% пациентов, у 40% пациентов отмечено улучшение контроля АГ более низкими дозами антигипертензивных препаратов [48]. В настоящее время обсуждается возможность односторонней адреналэктомии у пациентов с двусторонней локализацией ПГА с целью повышения эффективности медикаментозной терапии АГ [49].
При оценке остаточной секреции альдостерона с помощью теста с флудрокортизоном у 62% пациентов после односторонней адреналэктомии по поводу односторонней АПА наступила полная биохимическая ремиссия. У остальных пациентов отмечалась частичная нормализация показателей. Независимыми предикторами более благоприятного клинического прогноза были женский пол, молодой возраст, низкий индекс массы тела, длительность АГ≤6 лет, индекс латерализации по данным СВЗК>8 [3, 50].
Показательным в отношении эффективности проведенной операции и излечения АГ является проведение скрининга через 3 месяца после оперативного вмешательства. Конечный результат (активность ренина плазмы или концентрация прямого ренина, альдостерона, калия в плазме и АД) оценивают через 6–12 месяцев после адреналэктомии, в дальнейшем – ежегодно. Полная биохимическая ремиссия характеризуется коррекцией гипокалиемии (при наличии до операции), нормализацией уровня альдостерона, ренина плазмы и соответственно АРС. Об отсутствии биохимической ремиссии свидетельствует стойкая гипокалиемия (при наличии до операции) и/или стойкое повышение АРС; невозможность подавлять секрецию альдостерона в ходе послеоперационного подтверждающего теста. Клинически это проявляется сохранением исходных (до операции) цифр АД на фоне приема прежней антигипертензивной терапии [34].
Радикальное лечение микронодулярной и диффузной гиперплазии затруднительно в связи с отсутствием четкой локализации измененной ткани надпочечника. Потенциально возможным вариантом лечения является воздействие на источник гиперсекреции с помощью чрескожной термической аблации: процедура занимает мало времени, не требует госпитализации и общей анестезии [3].
Эффективность консервативной терапии во многом зависит от регулярности приема адекватно подобранных доз препарата в течение всей жизни, что сопряжено с риском развития осложнений, снижающих приверженность и переносимость лечения. Преобладание двусторонних форм ПГА и существующие сложности с проведением СВЗК являются факторами, из-за которых консервативная терапия является основным методом лечения ПГА.
В случаях, когда клиническая картина подозрительна в отношении ПГА, но отсутствует возможность проведения подтверждающих тестов, можно рассматривать пробное назначение консервативной терапии с дальнейшей оценкой эффекта. Спиронолактон является наиболее доступным, патогенетически оправданным выбором консервативной терапии ПГА. Являясь неселективным конкурентным антагонистом минералокортикоидных рецепторов, сходным по структуре с прогестероном, он действует как антагонист андрогеновых рецепторов и как агонист прогестероновых рецепторов, с чем связано развитие побочных эффектов у ряда пациентов, особенно при использовании дозы более 50 мг/сут. Такими проявлениями являются нарушения менструального цикла у женщин, развитие гинекомастии и снижение либидо у мужчин [49, 52]. В исследовании X. Jeunemaitre и G. Chatellier среди 699 мужчин, которым назначали спиронолактон в виде монотерапии или в сочетании с другим антигипертензивными препаратами, гинекомастия развилась в 91 случае (13%) и была обратимым, доза-зависимым эффектом. На фоне приема 50 мг и менее частота составила 6,9%, в то время, как доза 150 мг/сут и выше сопровождалась развитием гинекомастии в 52,2% [53].
В клинической практике медикаментозную терапию ПГА начинают со спиронолактона в дозе 12,5–25 мг/сут однократно, с титрацией до достижения концентрации калия в сыворотке 4,5 ммоль/л, повышения до определяемых значений подавленного ренина. В случае возникновения побочных эффектов возможен переход на селективный антагонист минералокортикоидных рецепторов – эплеренон, который значительно реже вызывает побочные эффекты. Это связано с тем, что аффинность связывания эплеренона с рецепторами андрогенов составляет 0,1% и <1% аффинности связывания с рецепторами прогестерона. По сравнению со спиронолактоном, у эплеренона более короткий период полужизни – этим обусловлена необходимость двукратного приема препарата, стартовая доза составляет 25 мг 2 раза в сутки. Результаты двойного слепого рандомизированного исследования демонстрируют, что Эплеренон менее эффективно снижает АД по сравнению со спиронолактоном, его терапевтическая доза составляет 200–300 мг/сут, для спиронолактона – 75–225 мг/сут [3, 20, 54]. Поскольку АГ у пациентов с ПГА является многофакторной и часто сочетается с эссенциальной гипертензией, для достижения контроля АД при необходимости целесообразно проведение многокомпонентной терапии. Оптимально добавлять калийсберегающие диуретики, блокаторы кальциевых каналов (БКК), гидрохлоротиазид, блокаторы рецептора ангиотензина-II (БРА) или ингибиторы ангиотензинпревращающего фермента (иАПФ). Добавление к терапии БКК оправданно с патогенетической точки зрения, поскольку соматические мутации в «генах – драйверах альдостерона» AПA прямо или косвенно усиливают внутриклеточную передачу сигналов кальция, экспрессии CYP11B2 и секреции альдостерона [52, 55]. Из этих мутаций 95% являются спорадическими, остальные 5% представляют собой семейные формы [56]. Спорадические соматические мутации чаще всего встречаются при АПА и диффузной гиперплазии. Значительная часть этих мутаций затрагивает гены, кодирующие ионные каналы, такие как калиевый канал KCNJ5, кальциевые каналы CACNA1D/CACNA1H, хлоридный канал CLCN1, АТФ-азы ATP1A1 и ATP2B3. Таким образом, БКК могут стать эффективным методом медикаментозной терапии ПГА. Высокие терапевтические дозы БКК, таких как верапамил, блокировали активность мутантного ионного канала KCNJ5 in vitro [57–59]. Еще одно исследование in vitro показало, что макролидные антибиотики (например, рокситромицин) избирательно ингибируют измененные каналы KCNJ5 [60]. Изучается потенциальная возможность их использования для предоперационной идентификации KCNJ5-положительной АПА. Существенным ограничением является соматическая природа данных мутаций, т.к. их затруднительно диагностировать без биопсии надпочечника.
К сожалению, даже при соблюдении всех рекомендаций лечение АМР не может полностью нивелировать действие альдостерона, не зависящее от минералокортикоидных рецепторов. Медикаментозная терапия с использованием новых селективных ингибиторов альдостеронсинтазы может стать эффективной альтернативой хирургическому вмешательству [61].
Заключение
Понимание новых аспектов патогенеза ПГА позволяет изменять подходы к диагностике, в т.ч. мягких форм заболевания, делать их более доступными для понимания и удобными в повседневной клинической практике. Будем надеяться, что упрощение алгоритмов сведет на нет прокрастинацию врачей всех специальностей, кто сталкивается с пациентами с АГ, в назначении исследований на альдостерон и ренин. Ясность понимания этапов диагностики, методов лечения и важности своевременной диагностики ПГА позволят снижать те безусловные риски, которые несет это заболевание.



