Введение
В настоящее время хроническая обструктивная болезнь легких (ХОБЛ) является одной из основных причин заболеваемости и смертности во всем мире среди лиц старше 40 лет [1, 2]. По мере прогрессирования заболевания у больных возникают обострения, снижение функции легких и физической активности, сопровождающееся ухудшением качества жизни (КЖ), инвалидизацией и смертностью [3, 4]. По данным Всемирной организации здравоохранения, ХОБЛ занимает третье место среди причин смерти во всем мире [1].
Представления о патофизиологической и фенотипической гетерогенности ХОБЛ в последние годы дополнено новыми подходами к этиологической классификации с введением понятия «этиотипы ХОБЛ» [1]. Целью нововведения является повышение осведомленности о возможных причинах ХОБЛ, не связанных с табакокурением, а также расширение представлений о соответствующих диагностических, профилактических и терапевтических стратегиях для множества этиотипов ХОБЛ, которые, по мнению ряда авторов, имеют распространенный характер [5].
Неизменными на протяжении последних десятилетий остаются две основных цели терапии ХОБЛ, тесно связанные и направленные в первую очередь на снижение выраженности симптомов, минимизацию влияния заболевания на повседневную деятельность и уменьшение риска будущих неблагоприятных событий, включающих обострения, прогрессирование заболевания и смертельные исходы [1, 6, 7].
На основании когортных исследований имеются убедительные доказательства того, что симптомы ХОБЛ оказывают значительное влияние на повседневную деятельность пациентов и их КЖ [8–10]. Кроме того, неадекватный контроль симптомов у больных ХОБЛ может приводить к увеличению заболеваемости и смертности [3, 11], сопровождаться ростом экономического бремени, связанного с более частыми госпитализациями и высокими затратами на стационарное лечение [12].
Среди наиболее важных факторов, определяющих социально-экономическое бремя ХОБЛ, рассматриваются тяжесть заболевания, наличие частых обострений и сопутствующих заболеваний, которые встречаются у 30–79% пациентов с ХОБЛ [9, 13]. Социально-экономическое бремя ХОБЛ считается значительным во всех странах и требует целевых ресурсов для оптимизации ведения больных ХОБЛ, включая контроль симптомов, профилактику обострений и эффективное лечение сопутствующих заболеваний [14].
Место бронходилататоров в терапии ХОБЛ
Ингаляционные бронходилататоры длительного действия (БДДД) в течение многих лет служат основой фармакологической терапии ХОБЛ при любой степени тяжести заболевания [1, 6, 7]. В последние годы позиционируется персонализированный подход к лечению больных ХОБЛ, при этом рекомендации по стартовой терапии и последующей коррекции лечения различаются в зависимости от клинических характеристик течения заболевания [4, 15].
В Федеральных клинических рекомендациях по ведению больных ХОБЛ БДДД определяются как основа терапии с необходимостью назначения комбинации БДДД в качестве старта при наличии выраженных симптомов ХОБЛ (одышка по шкале mMRC (Modified Medical Research Council) ≥2 или САТ (COPD Assessment Test) ≥10 баллов [6]. Начиная с 2023 г. версия международных рекомендаций GOLD (Global Initiative for Chronic Obstructive Lung Disease) также позиционирует назначение комбинации БДДД в качестве стартовой терапии для пациентов с выраженными симптомами (категория B) [1]. Несмотря на доказательства эффективности монотерапии БДДД при ХОБЛ [16], не менее половины пациентов, получающих это лечение, продолжают страдать от выраженных симптомов и обострений заболевания, что свидетельствует о недостаточности монотерапии для многих больных ХОБЛ [7, 9, 17].
Представление о характеристике тяжести ХОБЛ более 10 лет назад вышло за пределы оценки степени нарушения бронхиальной проходимости. Проведенные исследования показывают, что объем форсированного выдоха за первую секунду (ОФВ1) не отражает многогранной природы ХОБЛ и ее влияние на разные аспекты жизни пациентов [18]. Данный факт подтверждается низкой корреляционной зависимостью между ОФВ1 и симптомами ХОБЛ, а также другими пациент-ориентированными параметрами заболевания [19, 20].
Вместе с тем ОФВ1 по-прежнему сохраняет статус маркера тяжести обструктивных нарушений у больных ХОБЛ и имеет прогностическое значение [1, 4]. Кроме того, в крупномасштабных популяционных исследованиях показано, что наиболее эффективный контроль симптомов и обострений заболевания связан с улучшением спирометрических показателей у больных ХОБЛ [20, 21].
Несколько мета-анализов продемонстрировало превосходящее улучшение функции легких при использовании комбинаций длительно действующих бета-агониста и антихолинергического препарата (ДДБА/ДДАХП) по сравнению с монотерапией ДДАХП или ДДБА, в т.ч. в комбинации с ингаляционными глюкокортикостероидами (ИГКС/ДДБА) независимо от анамнеза обострений [22, 23]. Многочисленные рандомизированные контролируемые исследования (РКИ) свидетельствуют о ранних и устойчивых преимуществах на фоне терапии ДДБА/ДДАХП в отношении как клинико-функциональных показателей, так и параметров КЖ и частоты обострений [24, 25].
Более выраженный клинический эффект комбинации БДДД обусловлен одновременным фармакологическим воздействием на β2-адренорецепторы (β2-АР) и М-холинорецепторы (М-ХР) [26]. Обоснование аддитивных эффектов, наблюдаемых при комбинации БДДД, включает разные механизмы действия β2-агонистов и мускариновых антагонистов, а также потенциальные внутриклеточные взаимодействия на пути реализации этих механизмов [27]. В частности, ДДБА напрямую вызывают бронходилатацию, стимулируя β2-АР, тогда как добавление М-холинолитика может уменьшать бронхоконстрикторный эффект ацетилхолина, высвобождение которого модифицируется β2-агонистом [21]. Кроме того, некоторые исследования in vitro предполагают возможный синергетический, а не просто аддитивный эффект, когда два активных компонента вместе воздействуют на клеточную мишень, что позволяет оптимизировать бронходилатирующее действие и преодолевать вариабельность бронхомоторного тонуса, связанную с обструкцией дыхательных путей [27, 28]. Существующая перекрестная связь между подтипом М2-ХР и β2-АР инициирует ингибирующее влияние на высвобождение ацетилхолина, и это подтверждается тем, что блокада β2-АР при приеме β-блокаторов приводит к беспрепятственному усилению холинергической передачи с последующей бронхоконстрикцией и связанной с этим увеличением бронхиальной гиперреактивности (БГР). Соответственно, мускариновые антагонисты позволяют предотвращать острую бронхоконстрикцию и уменьшать БГР [29, 30].
Мишени бронходилатации у больных ХОБЛ
Интегральная исследовательская программа оценки эффективности комбинации ДДБА/ДДАХП, представленная индакатеролом/гликопирронием (ИНД/ГЛИ) в дозе 110/50 мг при однократном применении в сутки, – IGNITE (Indacaterol and GlycopyrroNium bromide clInical sTudiEs), состоящая из 11 РКИ продолжительностью от 6 до 64 недель с участием более 10 тыс. пациентов, показала существенное преимущество по увеличению ОФВ1 по сравнению с монотерапией индакатеролом, гликопирронием, тиотропием, а также комбинациями тиотропия и формотерола, салметерола/флутиказона [31, 32].
В частности, исследование SHINE, где первичной конечной точкой было значение минимального ОФВ1 (перед введением дозы исследуемого препарата), демонстрирует существенное улучшение при использовании ИНД/ГЛИ на 26-й неделе терапии по сравнению с монокомпонентами индакатеролом и гликопирронием: разница ОФВ1 составила 0,07 и 0,09 л соответственно, комбинация ИНД/ГЛИ превосходила тиотропий на 0,08 л и имела преимущество в 0,2 л по сравнению с плацебо (p<0,001 во всех случаях) [33].
Данные мета-анализа подтверждают преимущество ИНД/ГЛИ в увеличении ОФВ1 на 89 мл по сравнению с индакатеролом и гликопирронием, р<0,001 [23].
Результаты исследований ILLUMINATE/LANTERN (1263 больных ХОБЛ) по сравнению влияния как на значение минимального ОФВ1, так и на показатель ОФВ1 через 0–12 часов применения исследуемого препарата демонстрируют преимущество в 80–100 мл (p<0,001) на фоне 26-недельной терапии ИНД/ГЛИ по сравнению с салметеролом/флутиказоном независимо от степени тяжести обструктивных нарушений у больных ХОБЛ [34].
Объединенный анализ исследований по сравнению влияния ИНД/ГЛИ 110/50 мкг 1 раз в день и салметерола/флутиказона 50/500 мкг 2 раза в день на бронхообструкцию с участием 4617 пациентов в зависимости от статуса табакокурения продемонстрировал улучшение минимального ОФВ1 по сравнению с салметеролом/флутиказоном. Разница через 26 недель лечения составила 105 и 78 мл у текущих и бывших курильщиков соответственно (p<0,0001). При этом в группе больных ХОБЛ, продолжавших курить, применение ИНД/ГЛИ сопровождалось более выраженным эффектом: улучшение минимального ОФВ1 превышало 100 мл, что соответствует критерию минимальной клинически значимой разницы [35].
Синергетическое обоснование эффективности фиксированной комбинации БДДД демонстрирует исследование QUANTIFY, в котором прирост минимального ОФВ1 на фоне назначения ИНД/ГЛИ был на 68 мл больше (р<0,001) по сравнению со свободной комбинацией тиотропия и формотерола [36].
Улучшение показателей бронхиальной проходимости на фоне терапии ИНД/ГЛИ сопровождается значимым уменьшением выраженности одышки у пациентов с ХОБЛ. Так, исследование BLAZE демонстрирует существенное превосходство ИНД/ГЛИ в уменьшении одышки с достижением уровня клинически значимой разницы по шкале TDI (transition dyspnea index) через 6 недель терапии по сравнению с группой пациентов без БДДД – Δ1,37 балла (p<0,001), и существенное превосходство над тиотропием – Δ0,49 балла (р=0,021) [37]. Аналогичные данные по сравнению влияния на одышку ИНД/ГЛИ и тиотропия представлены в исследовании SHINE [33].
Данные двух мета-анализов подтверждают, что комбинация ДДАБ/ДДАХП, в частности ИНД/ГЛИ, способствует более выраженному уменьшению одышки по сравнению с монотерапией тиотропием, гликопирронием и индакатеролом [23, 38]. При этом вероятность достижения клинически значимого улучшения одышки по шкале TDI (≥1 балла) на фоне ИНД/ГЛИ выше на 19% по сравнению с тиотропием, а использование короткодействующих бронходилататоров по потребности меньше на 0,63 ингаляции в день (р<0,0001) [38]. При сравнении влияния на одышку использование ИНД/ГЛИ сопровождалось клинически значимым уменьшением одышки у 49,6% пациентов и существенно превосходило долю пациентов в группе нефиксированной комбинации тиотропия и формотерола – 42,4%, р=0,033 [36].
Объединенный анализ исследований ILLUMINATE/LANTERN по влиянию на одышку ИНД/ГЛИ и салметерола/флутиказона через 26 недель лечения демонстрирует разницу в уменьшении выраженности одышки на 0,47 балла по шкале TDI (p<0,05) в пользу ИНД/ГЛИ [34]. Представленные данные подтверждают преимущество ИНД/ГЛИ по улучшению бронхиальной проходимости и уменьшению выраженности симптомов по сравнению с монотерапией БДДД, ИГКС/ДДБА и нефиксированной комбинацией БДДД.
Показатели КЖ, оцененные с помощью вопросника госпиталя Святого Георгия (SGRQ), по данным исследовательской программы IGNITE на фоне применения ИНД/ГЛИ, как и другие клинико-функциональные параметры, существенно превосходили монотерапию БДДД [31, 39]. Аналогичная тенденция наблюдалась при сравнении ИНД/ГЛИ с ИГКС/ДДБА и нефиксированной комбинацией тиотропия и формотерола, однако статистически значимых различий по влиянию на параметры КЖ больных ХОБЛ при сравнении со свободной комбинацией БДДД не получено [34, 36].
Снижение частоты и тяжести обострений остается одной из основных целей лечения больных ХОБЛ [7]. Обострения ХОБЛ представляются важным компонентом характеристики клинического течения ХОБЛ, оказывая влияние на заболеваемость, смертность и нагрузку на систему здравоохранения [1, 6]. Кроме того, частые обострения ХОБЛ повышают риск прогрессирования заболевания [40] и являются определяющим фактором при выборе стартовой терапии ХОБЛ, ее последующей модификации [7, 41]. Результаты проведенных исследований подтверждают высокий превентивный потенциал ИНД/ГЛИ в снижении риска обострений ХОБЛ по сравнению не только с монотерапией БДДД [42], но и с комбинацией ИГКС/ДДБА [43]. В частности, в исследовании FLAME ИНД/ГЛИ превосходил салметерол/флутиказон в снижении годовой частоты всех обострений ХОБЛ на 11% (р=0,003) и на 17% (р<0,001) –
среднетяжелых и тяжелых обострений заболевания. Период времени до первого среднетяжелого или тяжелого обострения в группе больных, получавших ИНД/ГЛИ, был существенно больше и составлял 127 дней по сравнению с таковым у пациентов на терапии салметеролом/флутиказоном – 87 дней (р<0,001) [43]. При этом различия эффективности сравниваемых препаратов не зависели от уровня эозинофилов периферической крови. Как в подгруппе пациентов с количеством эозинофилов в крови менее 2%, так и у пациентов с количеством эозинофилов 2% и выше частота умеренных или тяжелых обострений была значительно ниже в группе ИНД/ГЛИ – 0,98 по сравнению с салметеролом/флутиказоном – 1,15 (р=0,01) [43, 44], что свидетельствует о снижении количества обострений в группе ИНД/ГЛИ независимо от уровня эозинофилов в крови. Следует отметить, что из исследования FLAME были исключены пациенты с высоким количеством эозинофилов в крови (более 600 клеток/мкл) и астмой в анамнезе. При этом частота пневмоний как ИГКС-ассоциированный эффект у больных ХОБЛ была закономерно выше в группе салметерола/флутиказона (4,8%) по сравнению с 3,2% в группе ИНД/ГЛИ (р=0,02) и не характеризовалась существенными различиями в зависимости от уровня эозинофилов [44]. Терапия ИНД/ГЛИ в течение 52 недель не только продемонстрировала превосходство в снижении частоты клинически значимых обострений ХОБЛ по сравнению с салметеролом/флутиказоном независимо от уровня эозинофилов, но и сопровождалась снижением вероятности пневмонии у пациентов с высоким риском обострения ХОБЛ.
При сравнении ИНД/ГЛИ с нефиксированной комбинацией тиотропия и формотерола не продемонстрировано статистически значимых различий по влиянию на частоту среднетяжелых и тяжелых обострений ХОБЛ в течение 26 недель исследования и времени их наступления [36].
Теоретическим обоснованием уменьшения обострений ХОБЛ на фоне терапии комбинацией БДДД, вероятнее всего, является совокупность патогенетически взаимосвязанных факторов, направленных на уменьшение выраженности легочной гиперинфляции (ЛГИ) и улучшение механики дыхания, снижение выработки бронхиального секрета и оптимизации мукоцилиарного клиренса, а также дополнительным противовоспалительным потенциалом ДДБА и ДДАХП, продемонстрированных in vitro и на животных моделях (рис. 1) [45].
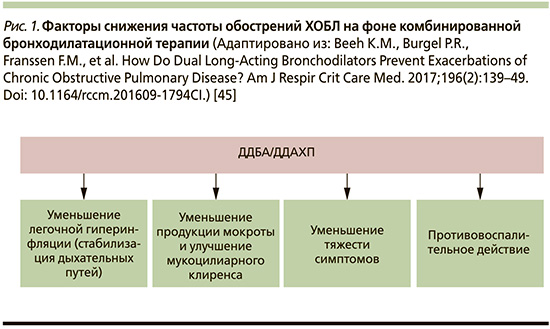
Таким образом, клинические исследования подтверждают фармакологические преимущества комбинации ДДБА/ДДАХП, демонстрируя превосходство над монотерапией в отношении улучшения функции легких, симптомов и КЖ больных, а также снижения частоты обострений ХОБЛ, по сравнению с монотерапией ДДБА или ДДАХ и комбинацией ИГКС/ДДБА.
ХОБЛ, бронходилататоры и сердечно-сосудистые заболевания
На естественную эволюцию и прогноз ХОБЛ влияют различные клинические факторы, при этом большое значение имеют сопутствующие заболевания [3, 15, 46]. Имеющиеся данные свидетельствуют, что наиболее частой коморбидной патологией у пациентов с ХОБЛ являются сердечно-сосудистые заболевания (ССЗ), отражая многокомпонентный и сложный патофизиологический кардиореспираторный континуум [47]. Помимо общих факторов риска, таких как курение, возраст, воспаление, в качестве связующих механизмов этих патологий могут рассматриваться нарушение бронхиальной проходимости и легочная гиперинфляция (ЛГИ) [46, 48, 49]. Кроме того, наличие и прогрессирование ССЗ является одним из пусковых факторов обострений ХОБЛ, тогда как обострения у пациентов с ХОБЛ могут провоцировать неблагоприятные сердечно-сосудистые события. В частности, вероятность инфаркта миокарда увеличивается в 2 раза в течение 5 дней обострения ХОБЛ, а распространенность инсульта увеличивается на 40% в течение первых 10 дней [50].
ССЗ являются не только наиболее частой и распространенной коморбидной патологией у больных ХОБЛ, но и одной из основных причин смертности среди них. Исследования последних лет показывают, что ССЗ являются второй по значимости причиной смертности среди больных ХОБЛ [51, 52]. Сочетание сердечно-сосудистого и бронхолегочного заболеваний значительно утяжеляет состояние больного и ухудшает прогноз. По данным недавно проведенного когортного исследования с участием 8020 пациентов, было показано, что риск смерти или тяжелого сердечно-сосудистого события существенно возрастает в первые недели после среднетяжелого/тяжелого обострения по сравнению с периодом стабильного течения заболевания: риск событий был выше в 15 раз (Hazard ratio [HR]=15,3, 95% доверительный интервал [ДИ]: 11,8–20,0) в 1–7-й дни, в 7 раз (HR=7,0, 95% ДИ: 4,9–10,0) – в 8–14-й дни после обострения и сохранялся повышенным примерно на треть (HR=1,3, 95% ДИ: 1,0–1,8) в 31–180-й дни после обострения ХОБЛ [53]. Представленные данные диктуют необходимость настороженности и применения эффективных терапевтических стратегий, направленных на снижение клинического и прогностического бремени сердечно-сосудистой коморбидности у больных ХОБЛ.
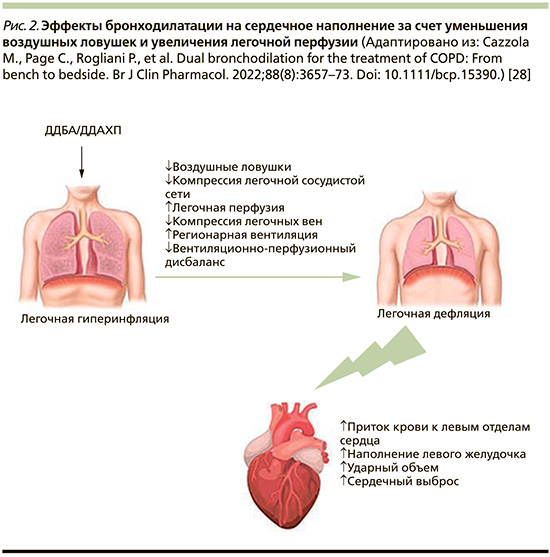
Установленным фактом является взаимосвязь между усилением степени тяжести обструктивных нарушений при ХОБЛ и увеличением риска смертности от ССЗ. Так, снижение ОФВ1 на 10% приводит к увеличению сердечно-сосудистой смертности на 28% [54]. Одним из механизмов, определяющих неблагоприятный исход ХОБЛ и ССЗ коморбидности, является ЛГИ. Гиперинфляция может играть важную роль в изменении размеров сердца и сердечной дисфункции у пациентов с ХОБЛ, что обусловлено уменьшением размеров камер сердца. Показатели ЛГИ (отношение объема вдоха к общей емкости легких, функциональная остаточная емкость и остаточный объем легких [ООЛ]) демонстрируют более сильную взаимосвязь с размерами камер сердца, чем обструкция дыхательных путей или диффузионная способность [49].
Исследование CLAIM, целью которого стала оценка влияния ИНД/ГЛИ на функцию сердца пациентов с ХОБЛ и ЛГИ, показало значительное снижение уровня ЛГИ, что привело к улучшению сердечной функции. Снижение ООЛ на 750 мл (p<0,0001) сопровождалось увеличением право- и левожелудочковых конечных диастолических объемов (КДО) (p≤0,0002) и улучшением сердечного выброса (p=0,0034) [55]. Последующий анализ исследования CLAIM, основанный на результатах динамической контрастной магнитно-резонансной томографии, свидетельствует не только о существенном улучшении регионарной вентиляции у больных ХОБЛ с ЛГИ на фоне терапии ИНД/ГЛИ, но и о существенном улучшении легочной микроциркуляции. Эти данные подтверждаются статистически значимой корреляционной зависимостью между показателями легочного микрососудистого кровотока и ООЛ (r=-0,34; р=0,012), КДО левого желудочка (r=0,35; р=0,011) и ударным объемом левого желудочка (r=0,44; р=0,001) [56]. Представленные данные позволяют сделать вывод: улучшение легочного микрососудистого кровотока, опосредованное дефляцией легких, является важным фактором, объясняющим патогенетическую взаимосвязь между ЛГИ и наполнением левого желудочка (рис. 2).
Профиль безопасности бронходилататоров при ХОБЛ остается первостепенным требованием терапии [57]. Анализ нежелательных явлений (НЯ) ИНД/ГЛИ в программе клинических исследований III фазы IGNITE продемонстрировал сопоставимый профиль безопасности при сравнении НЯ с монокомпонентами, тиотропием и комбинацией салметерола/флутиказона [33, 42, 58, 59]. Эти данные подтверждаются результатами объединенного анализа сравнения безопасности ИНД/ГЛИ и его монокомпонентов, тиотропия, плацебо у 11 404 пациентов всех завершенных РКИ III фазы продолжительностью ≥3 месяцев. Было отмечено, что комбинация ИНД/ГЛИ по сравнению с плацебо, монокомпонентами (индакатерол и гликопирроний) и тиотропием не увеличивала риск смерти, в т.ч. смертность от всех причин, серьезных НЯ сердечно-сосудистого и цереброваскулярного генеза, пневмонии, обострений ХОБЛ, а также не сопровождалась увеличением риска трепетания/фибрилляции предсердий среди всех больных ХОБЛ и в различных подгруппах. Так, результаты объединенного анализа показывают, что риск сердечно-сосудистых событий для ИНД/ГЛИ по сравнению с плацебо был сопоставимым между пожилыми (возраст ≥65 лет) и более молодыми пациентами, больными среднетяжелой и тяжелой ХОБЛ, а также при сравнении сердечно-сосудистой безопасности ИНД/ГЛИ у пациентов с низким и высоким рисками ССЗ (анамнез ССЗ, наличие гиперлипидемии, анамнез сахарного диабета II типа, ожирение [индекс массы тела >30 кг/м2], возраст ≥65 лет, текущий курильщик) [57]. Таким образом, эти данные обеспечивают определенный уровень уверенности в сердечно-сосудистой безопасности ИНД/ГЛИ для пациентов с ХОБЛ.
Заключение
Оценка клинической эффективности БДДД для больных ХОБЛ в последние десятилетия вышла за пределы их прямой фармакологической направленности и в большей степени определяется снижением ЛГИ, уменьшением клинической нагрузки на пациента и коморбидного обременения. Данные клинических исследований демонстрируют более высокую эффективность ИНД/ГЛИ в отношении функции легких, симптомов ХОБЛ и снижения частоты обострений по сравнению с монокомпонентами, тиотропием, ИГКС/ДДБА и нефиксированной комбинацией БДДД. Терапевтические возможности применения ИНД/ГЛИ при ХОБЛ свидетельствуют о существенном улучшении легочного микрососудистого кровотока и сердечной механики у больных ХОБЛ с гиперинфляцией легких. Полученные доказательства достижения основных клинических мишеней в терапии ХОБЛ и профиля безопасности, сопоставимого с плацебо, позволяют рассматривать ИНД/ГЛИ в качестве препарата для оптимальной стартовой и поддерживающей терапии ХОБЛ у большинства больных с выраженными симптомами, обострениями заболевания и сердечно-сосудистой коморбидностью.


