Введение
Хронический лимфолейкоз (ХЛЛ) – одно из наиболее часто встречающихся онкогематологических лимфопролиферативных заболеваний. На его долю приходится около 30% от всех лейкозов [1]. Являясь неизлечимым заболеванием, ХЛЛ характеризуется постоянным рецидивирующим течением, в связи с этим основной целью терапии является увеличение времени общей и безрецидивной выживаемости (ОВ и ВБП) и качества жизни пациента [2–4]. Выбор терапии в первую очередь определяется возрастом, а также числом и тяжестью сопутствующих заболеваний.
Согласно действующим российским [2, 5] и международным клиническим рекомендациям [4, 3], большинству пациентов с ХЛЛ в первой линии рекомендована химиоиммунотерапия, например FCR (флударабин, циклофосфамид и ритуксимаб) и BR (бендамустин и ритуксимаб). Однако при наличии факторов высокого риска, в частности наличия делеций 17р или мутаций ТР53, в отсутствие возможности проведения аллогенной трансплантации костного мозга препаратами выбора для таких пациентов становятся таргетные препараты или их комбинации с химиопрепаратами или между собой.
В настоящее время в Российской Федерации (РФ) в перечень жизненно необходимых и важнейших лекарственных препаратов включены следующие таргетные опции первой линии терапии ХЛЛ: ибрутиниб, акалабрутиниб венетоклакс в комбинации с обинутузумабом [6].
Ибрутиниб – пероральный ингибитор тирозинкиназы Брутона (иТКБ), первый таргетный препарат, зарегистрированный в РФ для лечения ХЛЛ. Ибрутиниб показал клиническую эффективность с длительным ответом [7–9] и превосходящие прежние опции терапии результаты в отношении ВБП у больных ХЛЛ, в т.ч. у взрослых пациентов с делецией 17р или мутацией ТР53, комплексным кариотипом, по сравнению со стандартными вариантами терапии [10].
Хотя непрерывный прием ибрутиниба является установленным стандартом лечения ХЛЛ, который обеспечивает улучшение выживаемости, растет потребность в удобном, полностью пероральном, ограниченном по времени варианте лечения, который можно принимать в амбулаторных условиях.
Венетоклакс, пероральный ингибитор антиапоптотического белка BCL-2, одобрен для лечения ХЛЛ в качестве монотерапии или в сочетании с ритуксимабом или обинутузумабом [11, 12].
Ибрутиниб и венетоклакс благодаря различным и взаимодополняющим механизмам действия влияют на отдельные клеточные компартменты и субпопуляции ХЛЛ, уничтожая как делящиеся, так и покоящиеся клетки опухоли [13, 14]. Ибрутиниб мобилизует клетки ХЛЛ из лимфатических узлов и других лимфоидных ниш в периферическую кровь, где они более восприимчивы к венетоклакс-индуцированному апоптозу, помимо этого ингибирование ТКБ ибрутинибом усиливает зависимость клеток ХЛЛ от BCL-2, тем самым повышая чувствительность к венетоклаксу и ускоряя апоптоз [13–16]. Комбинация ибрутиниба и венетоклакса продемонстрировала синергетическую противоопухолевую активность в доклинических моделях ХЛЛ, при этом при комбинации наблюдалась более высокая цитотоксичность, чем при применении любого из препаратов по отдельности [17].
CAPTIVATE (NCT02910583) – это международное многоцентровое исследование 2-й фазы, в котором изучали эффективность комбинированной терапии ибрутиниб+венетоклакс в первой линии ХЛЛ/ЛМЛ у пациентов в возрасте ≤70 лет в 2 отдельных когортах: рандомизированное прекращение лечения под контролем минимальной остаточной болезни (МОБ) и фиксированная длительность (ФД). В когорте МОБ неопределяемая МОБ была достигнута 75% пациентов в периферической крови и 68% в костном мозге после 3 циклов ибрутиниба, за которыми последовали 12 циклов ибрутиниба плюс венетоклакс, а 30-месячные показатели ВБП неизменно составляли ≥95% в течение последующих МОБ-контролируемых циклов терапии [18–20].
После публикации результатов первичного анализа когорты CAPTIVATE FD [20], показавших, что комбинация ибрутиниб плюс венетоклакс позволили добиться глубокого стойкого ответа и многообещающей ВБП, в т.ч. у пациентов с признаками высокого риска [20], ибрутиниб в комбинации с венетоклаксом признан оптимальной опцией терапии в первой линии терапии пациентов с ХЛЛ с комплексным кариотипом, согласно клиническим рекомендациям [5]: «Пациентам с верифицированным ХЛЛ/ЛМЛ с комплексным кариотипом независимо от возраста и коморбидности рекомендуется назначение комбинации венетоклакса и ибрутиниба ибрутиниб в дозе 420 мг/сут (3 капсулы) внутрь 1 раз в сутки в течение 3 циклов, венетоклакс в комбинации с ибрутинибом с 1-го дня 4 цикла ибрутиниба в режиме rump-up 20 мг/сут 1-я неделя, 50 мг/сут 2-я неделя; 100 мг/сут 3-я неделя; 200 мг/сут 4-я неделя; 400 мг/сут 5-я неделя и далее доза венетоклакса 400 мг/сут в комбинации с ибрутинибом в течение 12 циклов [18–20]».
В CAPTIVATE FD [20] удалось достичь полного ответа в 56% случаев (95% доверительный интервал [ДИ]: 48–64) у пациентов без del (17p), что значительно выше заранее установленного минимального уровня в 37% (р<0,0001). В общей группе частота полного ответа составила 55% (95% ДИ: 48–63); показатели неопределяемой МОБ составили 77% (периферическая кровь) и 60% (костный мозг).
Двадцатичетырехмесячная ВБП и ОВ составили 95 и 98% соответственно. При этом на исходном уровне 21% пациентов относились к категории высокого опухолевого бремени по риску синдрома лизиса опухоли, после введения ибрутиниба в этой категории остался только 1% пациентов. Наиболее частыми нежелательными явлениями (НЯ) степени ≥3 были нейтропения (33%) и артериальная гипертензия (6%).
Таким образом, ибрутиниб+венетоклакс первой линии представляет собой первую полностью пероральную схему приема 1 раз в сутки без химиотерапии для пациентов с ХЛЛ с максимальной на сегодняшний день клинической эффективностью.
Суммируя вышеизложенное, нынешняя терапия ХЛЛ предусматривает назначение комбинированной таргетной терапии пациентов с ХЛЛ высокого риска (del17p/mutTP53, non-mutIGHV и комплексным кариотипом [3 и более хромосомных аберраций]) уже в первой линии терапии, что делает проведение оценки экономических последствий (клинико-экономический анализ – КЭА и анализ влияния на бюджет) при использовании фиксированного режима комбинированной терапии ибрутиниб+венетоклакс в рамках льготного лекарственного обеспечения актуальным, что и явилось целью настоящего исследования.
Методы
В ходе настоящего исследования была построена аналитическая модель принятия решений в MS Excel, 2016, которая позволяет проводить КЭА при применении комбинации ибрутиниб+венетоклакс ФД по сравнению с применением FCR (флударабин, циклофосфамид и ритуксимаб) и анализ влияния на бюджет при применении комбинации ибрутиниб+венетоклакс ФД по сравнению с таргетной терапией (акалабрутиниб, ибрутиниб, комбинация венетоклакс+обинутузумаб) в терапии первой линии пациентов с ХЛЛ высокого риска (del17p/mutTP53, non-mutIGHV и комплексным кариотипом [3 и более хромосомных аберраций]).
При проведении анализа руководствовались следующими документами:
- Требования к методологическому качеству клинико-экономических исследований лекарственного препарата и исследований с использованием анализа влияния на бюджеты бюджетной системы РФ. Приложение № 5.1 к Правилам формирования перечней лекарственных препаратов для медицинского применения и минимального ассортимента лекарственных препаратов, необходимых для оказания медицинской помощи, утвержденным Постановлением Правительства РФ от 28.08.2014 № 2021 от 03.12.2020).
- Методические рекомендации по проведению сравнительной клиникоэкономической оценки лекарственного препарата, утвержденные Приказом ФГБУ «ЦЭККМП» Минздрава России от 29.12.2018 № 242-од.
- Методические рекомендации по оценке влияния на бюджет в рамках реализации программы государственных гарантий бесплатного оказания гражданам медицинской помощи, утвержденные приказом ФГБУ «ЦЭККМП» Минздрава России от 29.12.2018 № 242-од.
Анализ клинической эффективности сравниваемых альтернатив по выбранному показанию
В ходе первого этапа работы выполнен систематический поиск публикаций по результатам клинических исследований, оценивающих эффективность сравниваемых стратегий в первой линии терапии пациентов с ХЛЛ и комплексным кариотипом.
Было проведено сравнение следующих альтернативных вариантов ведения взрослых больных ХЛЛ с неблагоприятным прогнозом заболевания, включая пациентов с немутированным статусом IGHV генов:
1. Ибрутиниб+венетоклакс по сравнению с FCR.
2. Ибрутиниб+венетоклакс по сравнению с таргетной терапией (акалабрутиниб, ибрутиниб, комбинация венетоклакс+обинутузумаб).
В клинических исследованиях для оценки тяжести заболевания и реакции на проводимое лечение используется несколько критериев эффективности, однако наиболее актуальным для оценки затрат системы здравоохранения остаются выживаемость без прогрессирования (ВБП), общая выживаемость (ОВ), общий ответ (ОО), частота контроля заболевания. Критерием безо-пасности сравниваемых схем терапии служила частота развития НЯ 3–4-й степеней.
Был выполнен поиск мета-анализов, рандомизированных клинических испытаний (РКИ) и непрямых сравнений. Поиск был выполнен в базе данных (БД) Medline (https://www.ncbi.nlm.nih.gov/pubmed) и Кокрановской библиотеки (https://www.cochranelibrary.com) в соответствии со следующей методикой:
Дата осуществления поиска: 28.08.2022; описание поиска № 1: поисковый запрос:
#1: («chronic lymphocytic leukaemia» [All Fields] OR «leukemia, lymphocytic, chronic, b cell»[MeSH Terms] OR («leukemia» [All Fields] AND «lymphocytic» [All Fields] AND «chronic» [All Fields] AND «b cell» [All Fields]) OR «b-cell chronic lymphocytic leukemia»[All Fields] OR («chronic» [All Fields] AND «lymphocytic»[All Fields] AND «leukemia» [All Fields]) OR «chronic lymphocytic leukemia»[All Fields])
#2: («ibrutinib»[Supplementary Concept] OR «ibrutinib»[All Fields] OR «ibrutinib s»[All Fields])) AND («venetoclax»[Supplementary Concept] OR «venetoclax»[All Fields]) OR («fludarabin»[All Fields] OR «fludarabine» [Supplementary Concept] OR «fludarabine» [All Fields])) AND («cyclophosphamide» [MeSH Terms] OR «cyclophosphamide» [All Fields] OR «cyclophosphamid» [All Fields] OR «cyclophosphamide s» [All Fields] OR «cyclophosphamides» [All Fields]) AND («rituximab»[MeSH Terms] OR «rituximab» [All Fields] OR «rituximab s»[All Fields]) AND («clinical trial» [Publication Type] OR «meta analysis» [Publication Type] OR «randomized controlled trial»[Publication Type] OR «review»[Publication Type] OR «systematic review»[Filter]) AND («clinical trial» [Publication Type] OR «meta analysis» [Publication Type] OR «randomized controlled trial» [Publication Type] OR «systematic review»[Filter]) AND (clinicaltrial[Filter] OR meta-analysis[Filter] OR randomizedcontrolledtrial [Filter] OR systematicreview [Filter])
#3: #1 AND #2
В обеих БД по указанному поисковому запросу найдено 155 статей, из которых были отобраны РКИ, ретроспективные исследования и мета-анализы.
Поскольку прямых сравнительных РКИ сравниваемых стратегий терапии обнаружено не было в качестве источника данных о клинической эффективности и безопасности сравниваемых стратегий приняты данные о ВБП и ОВ у пациентов с ХЛЛ с немутированным статусом IGHV генов для 3-летнего наблюдения когорты ФД исследования CAPTIVATE [20, 21] или 5-летнего наблюдения когорты FCR в исследовании E1912 [22, 23]. Режимы назначения препаратов в данных РКИ соответствовали клиническим рекомендациям в РФ [5].
Тридцатишестимесячная ВБП для ибрутиниб+венетоклакс у пациентов с ХЛЛ с немутированным статусом IGHV генов составила 86%, 36-месячная ОВ – 97% соответственно [20, 21], 60-месячная ВБП для FCR у пациентов с ХЛЛ с немутированным статусом IGHV генов составила 33%, 60-месячная ОВ – 84% соответственно [22, 23].
Далее был выполнен систематический поиск публикаций по результатам клинических исследований, оценивавших эффективность иТКБ у пациентов с ХЛЛ. Прямых сравнительных РКИ, мета-анализов и непрямых сравнений для сравниваемых стратегий терапии обнаружено не было. Вследствие этого на основании данных скорректированного непрямого сравнения S. Matthew et al. (2021) [24] приняли допущение о равнозначной эффективности всех сравниваемых альтернатив. За основу сравнения были взяты РКИ RESONATE-2 (ибрутиниб в первой линии терапии ХЛЛ) [25] в ELEVATE TN (акалабрутиниб в первой линии лечения ХЛЛ) [26], CLL-14 (венетоклакс+обинутузумаб в 1-й линии терапии ХЛЛ) [12]. Не было показано статистически значимой разницы в ВБП между препаратами сравнения.
Несмотря на то что в CAPTIVATE FD [20] 36-месячная ВБП и ОВ составили 95 и 98% соответственно, что говорит о потенциально более высокой эффективности данной комбинации, в условиях отсутствия непрямых сравнений принято допущение о равной эффективности таргетной терапии (табл. 1).

Описание модели
Расчеты показателей проводились на основании модели, построенной в программе Microsoft Excel, 2016. Для моделирования исходов лечения была предложена математическая модель прогрессирования ХЛЛ с неблагоприятным прогнозом заболевания, включившая пациентов с немутированным статусом IGHV генов в случае использования каждого из рассматриваемых вариантов сравнения. Математическая модель (рис. 1) предусматривает следующие последовательные взаимоисключающие состояния, в которых могут находиться пациенты:
1. Без прогрессирования.
2. Прогрессия.
3. Смерть.
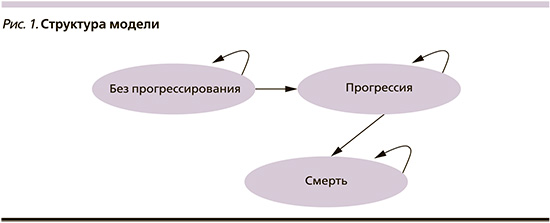
Для целей КЭА был выбран пожизненный временной горизонт исследования (но не более 100 лет) с шагом моделирования 1 неделя.
Вероятность переходов между состояния рассчитывалась на основании данных о ВБП и ОВ у пациентов с ХЛЛ с немутированным статусом IGHV генов для 3-летнего наблюдения когорты ФД исследования CAPTIVATE [20, 21] или 5-летнего наблюдения когорты FCR в исследовании E1912 [22, 23]. Тридцатишестимесячная ВБП для ибрутиниб+венетоклакс у пациентов с ХЛЛ с немутированным статусом IGHV генов составила 86%, 36-месячная ОВ – 97% соответственно [20, 21], 60-месячная ВБП для FCR у пациентов с ХЛЛ с немутированным статусом IGHV генов составила 33%, 60-месячная ОВ – 84% соответственно [22, 23].
Все пациенты начинали цикл в состоянии «Без прогрессирования», где в варианте «ибрутиниб+венетоклакс» или «FCR» проводится терапия с применением одноименного лекарственного препарата. В каждом последующем шаге модели пациенты могут остаться в данном состоянии или перейти в состояние «Прогрессия», в котором, согласно клиническим рекомендациям, им назначается монотерапия акалабрутинибом (для группы «ибрутиниб+венетоклакс») или ибрутинибом (для варианта терапии «FCR») или «Смерть», в котором пациенты выбывают из моделирования.
В отсутствие прогрессирования использовался сценарий с терапией ФД I+V и 6 циклами FCH, согласно данным T.D. Shanafelt et al. [22].
Модель предусматривает расчет показателя, характеризующего результативность лечения: число дополнительно прожитых лет жизни без прогрессирования за период моделирования. Расчет данного показателя за период моделирования T осуществлялся при помощи следующей формулы:
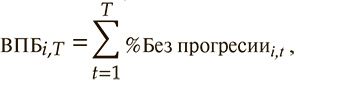
где %Lifei,t – доля пациентов, находящихся в состоянии «Без прогрессии» в среднем за период времени t при использовании варианта сравнения i.
Для учета затрат на стоимость годового курса терапии цена рассчитывалась на всех пациентов в среднем за каждый период моделирования. Поскольку при проведении анализа эффективности была выявлена различная клиническая эффективность сравниваемых стратегий терапии, при проведении собственно фармакоэкономического анализа применен анализ эффективности затрат (CEA – cost-effectiveness analysis) с расчетом соответствующего коэффициента (CER – cost-effectiveness ratio) с помощью следующей формулы:
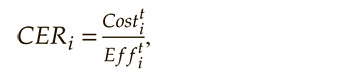
где CERi – соотношение «затраты/эффективность» при применении варианта терапии i;
Cost – средние расходы системы обязательного медицинского страхования на одного пациента за t-период при применении соответствующего варианта терапии i;
Eff – значение критерия эффективности, измеренного за t-период при применении варианта терапии i.
Анализ влияния на бюджет
В соответствии с п. 5.2.2 «Методических рекомендаций по оценке влияния на бюджет в рамках реализации программы государственных гарантий бесплатного оказания гражданам медицинской помощи» и сроком формирования бюджета здравоохранения анализ «влияния на бюджет» (АВБ) выполнялся с годичной и 5-летней временнóй перспективой по следующим формулам:
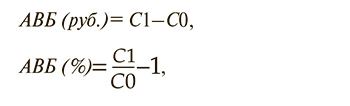
где АВБ (руб.) – разница в суммарных затратах между текущим вариантом лекарственной терапии и ожидаемым (с использованием исследуемого лекарственного препарата), руб.;
АВБ (%) – разница в суммарных затратах между текущим вариантом лекарственной терапии и ожидаемым (с использованием исследуемого лекарственного препарата), %;
С0 – суммарная стоимость терапии всех пациентов при базовом распределении;
С1 – стоимость терапии при потенциальном увеличении количества пациентов, использующих ибрутиниб+венетоклакс.
При расчете затрат, начиная со 2-го года в модели, было учтено дисконтирование затрат при использовании ставки дисконтирования, равной 5% по следующей формуле:

Costs – недисконтированные затраты;
i – ставка дисконтирования;
t – период дисконтирования (в годовом выражении).
Численность целевой популяции для проведения АВБ была рассчитана на основании данных, представленных в клинических рекомендациях, статистических и эпидемиологических исследованиях. При этом из пациентов с ХЛЛ выделена группа пациентов с неблагоприятным прогнозом заболевания, включая пациентов с немутированным статусом IGHV генов. Данная группа характеризуются плохим ответом на терапию препаратами, в т.ч. включенными в Перечень дорогостоящих лекарственных препаратов и низкой выживаемостью. Среди всех пациентов с ХЛЛ доля пациентов с немутированным статусом IGHV генов составляет 63% [30].
Ибрутиниб в комбинации с венетоклаксом является препаратом выбора в практике терапии ХЛЛ в первой линии пациентов со следующими характеристиками [5]:
- Имеются показания к лечению по критериям IwCLL.
- Не имеется делеции 17p/мутации TP53.
- Немутированный статус IGHV.
По оценке экспертов, заболеваемость ХЛЛ составляет около 2799 человек в год, примерно у 74% (2071 пациент) имеются показания к лечению, 93% (1926 пациентов) из них не имеют делеции 17p/мутации TP53 [5]. На текущий момент в рамках Программы высокозатратных нозологий (ВЗН) для данных пациентов доступна терапия режимом FCR. При этом в соответствии с актуальными данными, накопленными в ходе клинических исследований, среди вышеуказанных категорий пациентов можно выделить группу с неблагоприятным прогнозом заболевания (немутированным статусом IGHV генов), составляющих около 63% (1214 пациентов) от вышеуказанных категорий [3]. Данная группа характеризуются плохим ответом на терапию препаратами, в т.ч. включенными в Перечень дорогостоящих лекарственных препаратов, и низкой выживаемостью. Одной из наиболее эффективных для этих пациентов представляется таргетная терапия [2].
Распределение препаратов взято из данных мониторинга IQVIA (2022). В качестве гипотезы при проведении АВБ принято следующее: доли ибрутиниба в монотерапии, акалабрутиниба и венетоклакса в комбинации с обинутузумабом замещались ибрутинибом в комбинации с венетоклаксом на горизонте 5 лет.
Анализ затрат
В модели были учтены прямые медицинские затраты на одного пациента, включая следующие виды затрат:
- затраты на лекарственную терапию, а именно на лекарственные препараты (ЛП) в составе сравниваемых схем.
Расчет стоимости курса
Для расчета затрат на лекарственную терапию были использованы режимы дозирования, рекомендованные в инструкциях к применению препаратов.
- Ибрутиниб в комбинации с венетоклаксом в первой линии терапии ХЛЛ назначается по следующей схеме: ибрутиниб в дозе 420 мг/сут (3 капсулы) внутрь 1 раз в сутки в течение 3 циклов, венетоклакс в комбинации с ибрутинибом с 1 дня 4 цикла ибрутиниба в режиме rump-up 20 мг/сут 1-я неделя, 50 мг/сут 2-я неделя, 100 мг/сут 3-я неделя, 200 мг/сут 4-я неделя, 400 мг/сут 5-я неделя и далее – доза венетоклакса 400 мг/сут в комбинации с ибрутинибом в течение 12 циклов [5].
- FCR в первой линии терапии ХЛЛ применяется по следующей схеме: флударабин 40 мг/м2 внутрь, дни 1–3 [31]; циклофосфамид 250 мг/м2 внутрь, дни 1–3; ритуксимаб – 1 цикл: 375 мг/м2 в/в кап., день 1, последующие циклы: 500 мг/м2 в/в кап., или 1600 мг п/к, день 1 [2]. Лечение возобновляется на 29-й день. При расчете предполагали, что пациентам будет назначено 6 циклов терапии FCR [2].
- Ибрутиниб в монотерапии, согласно инструкции, назначается в дозе 420 мг (3 капсулы по 140 мг) 1 раз в сутки.
- Акалабрутиниб, согласно инструкции, назначается в дозе 100 мг 2 раза в сутки.
- Венетоклакс в комбинации с обинутузумабом в первой линии терапии ХЛЛ применяется по следующей схеме: венетоклакс 20 мг/сут дни 22–28 цикла 1; 50 мг/сут дни 1–7 цикла 2; 100 мг/сут дни 8–14 цикла 2; 200 мг/сут дни 15–21 цикла 2; 400 мг/сут дни 22–28 цикла 2, далее 400 мг/сут до последнего дня 12-го цикла и обинутузумаб 100 мг, день 1 цикла 1; 900 мг день 2 цикла 1; 1000 мг день 8 цикла 1; 1000 мг день 15 цикла 1; 1000 мг день 1 цикла 2; 1000 мг/сут день 1 циклов 3–6. ФД 12 циклов по 28 дней каждый.
В данном исследовании стоимость лекарственных препаратов сравнения определялась по данным Государственного реестра предельных отпускных цен – ГРЛС (расчет проводился 20.09.2022).
В ГРЛС на момент расчета представлено 6 реестровых записей для международного непатентованного наименования ибрутиниб. Согласно данным, доступным в Реестре изобретений РФ [32] и в Реестре евразийских патентов [33], патентная защита референтного ЛП с торговым наименованием «Имбрувика» РУ № ЛП-002811 (МНН ибрутиниб) продолжит действовать по меньшей мере до 2028 г. Обращение на территории РФ воспроизведенного ЛП с торговым наименованием «Ибрутиниб-натив» РУ № ЛП-006261 (МНН ибрутиниб) в период до окончания патентной защиты лекарственного препарата «Имбрувика» является нарушением патентных прав в силу ст. 1229, 1358 Гражданского кодекса РФ. Также, согласно данным анализа электронных аукционов по закупке ЛП для государственных и муниципальных нужд [34] за период январь – август 2022 г., закупок ЛП «Ибрутиниб-натив» РУ № ЛП-006261 не осуществлялось. Соответственно, цена на ЛП «Ибрутиниб-натив» была исключена из расчета. С учетом вышеописанного подхода медиана стоимости ЛП ибрутиниб 140 мг № 90 (без учета НДС) составляет 385 505,82 руб. (или 30,60 руб. за 1 мг).
При расчете стоимости терапии дополнительно учитывался НДС (10%). Стоимость ритуксимаба определялась как расчетная медиана стои-мости всех торговых наименований ритуксимаба. Согласно принятому допущению, средняя площадь поверхности тела для расчета стоимости терапии ритуксимабом определена равной 1,82 м2 (табл. 2).
При проведении анализа «затраты–эффективность» использовали только данные о стоимости полнодозовой терапии до прогрессии с учетом допущения о равной частоте отказов от терапии.
Анализ чувствительности
Для изучения влияния изменчивости параметров разработанной модели на результаты моделирования проведен анализ чувствительности. В качестве изменяющихся параметров выступали цены на ЛП, численность целевой популяции пациентов и численность (доля) пациентов, получающих предлагаемый ЛП.
Результаты
Анализ затрат, оценка стоимости курса лекарственной терапии
Результаты оценки стоимости годового курса терапии из расчета на одного пациента представлены в табл. 3.
Анализ «затраты–эффективность»
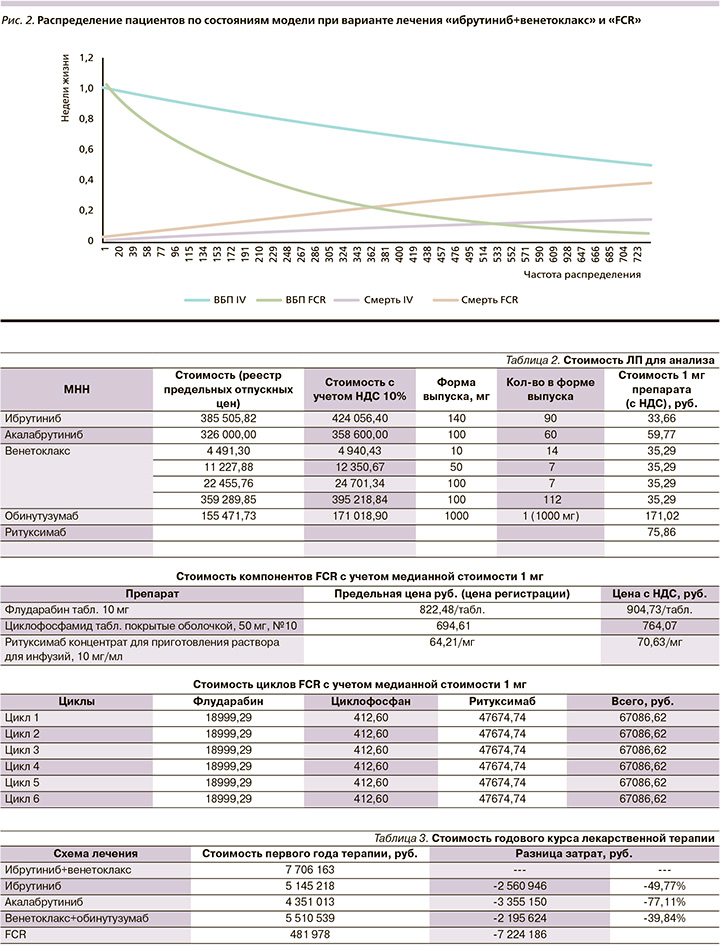
Распределение пациентов по состояниям модели в течение их последующей жизни для вариантов «ибрутиниб+венетоклакс» и «FCR» представлено на рис. 2
Среднее число прожитых лет без прогрессирования в течение 20-летнего периода моделирования при ХЛЛ при использовании ибрутиниба в комбинации с венетоклаксом составит 12,63 года, что на 8,16 года больше, чем среднее число лет без прогрессирования, которые проживет один пациент, если он будет получать FCR, – 4,47 года.
Среднее число прожитых лет в течение 20-летнего периода моделирования при ХЛЛ при использовании ибрутиниба в комбинации с венетоклаксом составит 18,12 года, что на 3,7 года больше, чем среднее число лет, которые проживет один пациент, если он будет получать FCR, – 14,41 года.
Результаты анализа «затраты–эффективность» для применения рассматриваемых вариантов сравнения представлены в табл. 4.
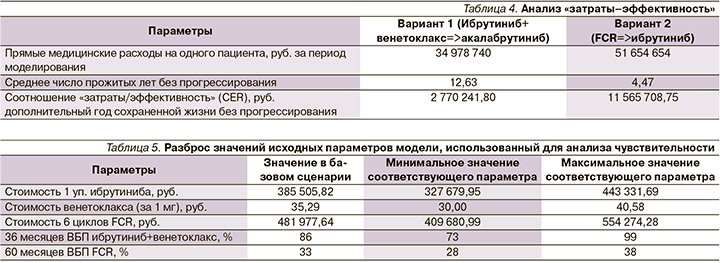
Согласно полученным результатам, применение ибрутиниба в комбинации с венетоклаксом позволяет увеличивать продолжительность жизни без прогрессирования взрослых больных ХЛЛ с неблагоприятным прогнозом заболевания, включая пациентов с немутированным статусом IGHV генов на 8,16 года за 20 лет моделирования, стоимость дополнительного года жизни без прогрессирования составляет 2 770 241,80 руб/год. Метод является доминантным с экономической точки зрения, т.к. коэффициент «затраты–эффективность» по показателю «год жизни без прогрессирования» для ибрутиниба с венетоклаксом ниже, чем для FCR.
Стоит отметить, что затраты на терапию FCR с назначением ибрутиниба при прогрессировании становятся дороже терапии ибрутиниб+венетоклакс с назначением акалабрутиниба при прогрессировании уже через 7,4 года моделирования.
Анализ чувствительности результатов анализа «затраты–эффективность»
Для оценки устойчивости полученных результатов проведен анализ чувствительности КЭА. Как было указано ранее, уровень неопределенности был равен 15%. В анализе учтены изменения цен на ЛП, эффективность ЛП. В качестве параметра эффективности рассматривалась разница показателей CER. В табл. 5 приведен разброс значений исходных параметров, использованный для анализа чувствительности. Результаты анализа чувствительности приведены в табл. 6.
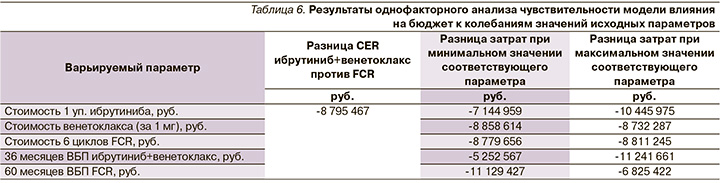
Наибольшее влияние на разницу показателей CER (ибрутиниб+венетоклакс против FCR) оказывает изменение эффективности и стоимости ибрутиниба. Результаты КЭА устойчивы к изменению вводных параметров (изменение эффективности и стоимости ЛП), поскольку разница CER при изменении всех вышеперечисленных параметров остается в пользу ибрутиниба+венетоклакса.
Анализ «влияния на бюджет»
Результаты анализа влияния на бюджет приведены в табл. 7.
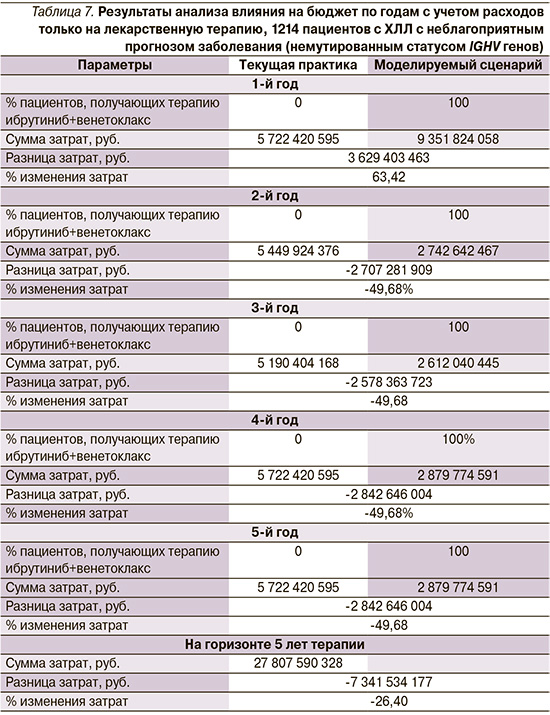
Результаты анализа влияния на бюджет применения ибрутиниб+венектоклакс в первой линии терапии пациентов с ХЛЛ с неблагоприятным прогнозом заболевания показали, что несмотря на более высокую стоимость терапии в 1 год, за счет ФД терапии нагрузку на бюджет системы здравоохранения удастся снизить до 26,4%, или на 7 342 млн руб., за 5 лет терапии при учете только затрат на ЛП.
При этом комбинация ибрутиниба с венетоклаксом сохраняет экономическое преимущество и по сравнению с опциями таргетной терапии ХЛЛ второй линии терапии (табл. 8).
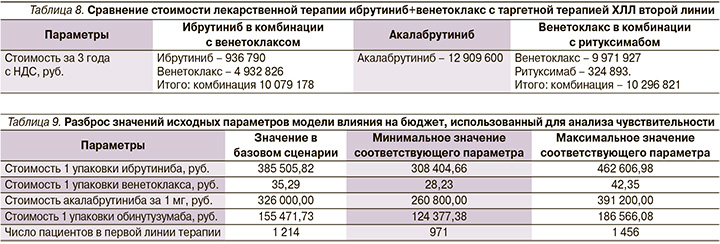
Анализ чувствительности результатов АВБ к изменениям исходных параметров для целевой популяции пациентов и ценам на ЛП
Для оценки устойчивости полученных результатов анализа влияния на бюджет проведен анализ чувствительности. Уровень неопределенности был равен 20% (для изменения цен на ЛП и численности целевой популяции). Таким образом, выбранные параметры были изменены на ±20% от базового уровня (детерминированного уровня). В анализе учтены изменения цен на ЛП и число пациентов. В табл. 9 приведен разброс значений исходных параметров, использованный для анализа чувствительности. Результаты анализа чувствительности приведены в табл. 10.
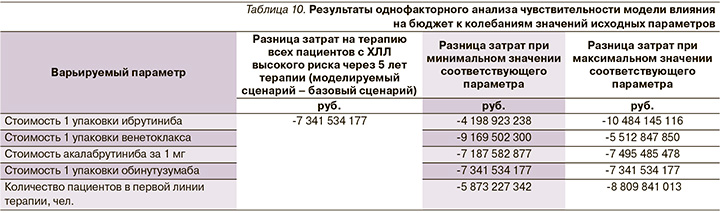
Наибольшее влияние на результаты анализа оказывает изменение стоимости ибрутиниба и венетоклакса. Изменение числа пациентов в диапазоне от +20% до -20% было ассоциировано со снижением прямых медицинских затрат. Результаты анализа чувствительности показали снижение прямых медицинских затрат во всех сценариях.
Фармакологистические аспекты использования схемы ибрутиниб+венетоклакс
В рамках фармакологистики – новой клинико-фармакологической парадигмы [35], ранжирующей ЛП в соответствии с факторами, определяющими их ценность для стейкхолдеров системы здравоохранения и общества в целом, обсуждаемая схема ибрутиниб+венетоклакс в лечении пациентов с ХЛЛ представлена следующими характеристиками:
- Ценность для пациентов. В обсуждаемом кейсе она является определяющей и наиболее очевидной: 1) среднее число прожитых лет и среднее число прожитых лет без прогрессирования в течение 20-летнего периода существенно выше, чем при использовании FCR; 2) возможность повышения эффективности лечения (увеличения продолжительности жизни без прогрессирования) у взрослых больных с неблагоприятным прогнозом заболевания, включая пациентов с немутированным статусом IGHV генов. В случае включения комбинации в клинические рекомендации для лечения пациентов с ХЛЛ в первой линии ее ценность для пациентов еще более возрастет (ввиду расширения применения в клинической практике).
- Ценность для государства/системы здравоохранения. Она представлена доказанными фармакоэкономическими преимуществами 1) за счет меньшей стоимости схемы ибрутиниб+венетоклакс для системы здравоохранения по сравнению с FCR, начиная с 7-летнего горизонта; 2) ввиду более низкого коэффициента «затраты–эффективность» по показателю «год жизни без прогрессирования» для ибрутиниба с венетоклаксом, чем для FCR; 3) за счет существенного снижения влияния на бюджет по сравнению с другими препаратами таргетной терапии на 5-летнем горизонте моделирования). Эта дополнительная ценность проявляется за рамками годовых бюджетов первых нескольких лет и становится значимой при оперировании 5–7-летними временными промежутками, что характерно в случае лечения пациентов с ХЛЛ, длительно получающими терапию.
Заключение
За счет ФД и высокой эффективности схема ибрутиниб+венетоклакс фармакоэкономически наиболее выгодна:
- Среднее число прожитых лет без прогрессирования в течение 20-летнего периода моделирования при ХЛЛ при использовании ибрутиниба в комбинации с венетоклаксом составит 12,63 года, что на 8,16 года больше, чем среднее число лет без прогрессирования, которые проживет один пациент, если он будет получать FCR, – 4,47 года.
- Среднее число прожитых лет в течение 20-летнего периода моделирования при ХЛЛ при использовании ибрутиниба в комбинации с венетоклаксом составит 18,12 года, что на 3,7 года больше, чем среднее число лет, которые проживет один пациент, если он будет получать FCR, – 14,41 года.
- По сравнению с включенным в программы ВЗН режимом терапии (FCR) уже на 7-летнем горизонте стоимость схемы ибрутиниб+венетоклакс для системы здравоохранения становится ниже стоимости FCR за счет ФД при превосходящих показателях эффективности.
- Применение ибрутиниба в комбинации с венетоклаксом позволяет увеличивать продолжительность жизни без прогрессирования взрослых больных ХЛЛ с неблагоприятным прогнозом заболевания, включая пациентов с немутированным статусом IGHV генов, на 8,16 года за 20 лет моделирования, стоимость дополнительного года жизни без прогрессирования составляет 2 770 241,80 руб/год. Метод является доминантным с экономической точки зрения, т.к. коэффициент «затраты–эффективность» по показателю «год жизни без прогрессирования» для ибрутиниба с венетоклаксом на 7 341 534 177 ниже, чем для FCR.
- По сравнению с другими препаратами таргетной терапии ХЛЛ нагрузку на бюджет системы здравоохранения при использовании ибрутиниб+венетоклакс удастся снизить до 26,4%, или на 7 342 млн руб., за 5 лет терапии
Таким образом, ибрутиниб+венетоклакс является доминантным методом, т.к. при большей эффективности обладает меньшей стоимостью по сравнению с текущей опцией терапии в рамках ВЗН и с другими таргетными опциями терапии ХЛЛ.
Вклад авторов. С.В. Недогода, А.С. Саласюк – разработка модели, анализ и интерпретация результатов, редактирование, финальное утверждение рукописи. А.С. Саласюк, И.Н. Барыкина, В.О. Лутова, Е.А. Попова – написание и редактирование текста статьи, проверка и утверждение текста статьи.



