Введение
Инъекционный липолиз (син.: липотерапия) представляет собой метод введения определенных медицинских растворов прицельно в жировую ткань с целью разрушения жировых клеток и уменьшения ее объема путем расщепления жира до глицерина и жирных кислот. Этот метод давно находится в центре внимания врачей эстетической медицины. Методику введения липолитиков в подкожно-жировую клетчатку с целью липолиза начали применять в середине 1980-х гг. в Италии. За те годы эффект от проведения липотерапии был подтвержден многочисленными клиническими наблюдениями.
Мировой опыт инъекционного липолиза
Частота и особенности применения инъекционного липолиза сильно различаются в разных странах. Процедура запрещена к применению в Бразилии и Турции, также ее популярность резко сократилась в США из-за развития осложнений. В Европе инъекционный липолиз востребован в Австрии, Германии и Великобритании. Стремление избегать хирургического вмешательства в азиатских странах также популяризирует этот метод. Сообщается, что в Корее инъекционный липолиз более популярен, чем инъекции ботулотоксина. Корейские врачи популяризируют инъекционный липолиз как экономически выгодную процедуру коррекции объемов тела [1].
Классификация липолитиков
Достаточно часто в российских публикациях встречаются упоминания «прямые липолитики» и «непрямые липолитики» [2, 3].
Прямые липолитики – препараты, действие которых направлено на разрушение жировых клеток и их мембран. К ним относят фосфатидилхолин (ФХ) и дезоксихолат натрия (ДХ).
Непрямые липолитики – препараты для локальной активизации обмена веществ и стимуляции распада липидов в жировых клетках. К этой группе относят аминофиллин, теофиллин, кофеин, изопротеренол, карнитин, пируват кальция, йохимбин, экстракт артишока и др. [4].
Механизм действия базовых препаратов
Основу большинства липолитиков составляют два базовых вещества – дезоксихолевая кислота и ФХ.
ФХ – собирательный термин для группы родственных соединений из класса фосфолипидов. В их состав кроме глицерина и жирных кислот входят фосфорная кислота и холин. Для ФХ организма человека характерно присутствие насыщенных жирных кислот – пальмитиновой, стеариновой и олеиновой. Для фармацевтических целей ФХ выделяют из соевых бобов. В его состав входят преимущественно ненасыщенные жирные кислоты – линолевая и aльфа-линоленовая. ФХ обеспечивает эмульгирование триглицеридов уже во внеклеточном пространстве с образованием жировых капель, которые в дальнейшем подвергаются стандартному процессу фагоцитоза.
Также ФХ представляет собой соединение, из которого организм вырабатывает ацетилхолин и поверхностноактивное вещество для альвеол легких. Образование камней в желчном пузыре, жировая болезнь печени и фиброз также связаны с ФХ [5, 6]. Кроме того, предварительные данные свидетельствуют, что он может играть роль в неврологических, эндокринных и психических расстройствах [7–9].
С целью изучения эффективности метода проведено исследование 441 пациента, которым вводили смесь ФХ и ДХ. Это исследование продемонстрировало, что липолитическая терапия может успешно использоваться в лечении локальных жировых отложений в области живота, бедер, ягодиц, предплечий и лица, а также жировых пакетов в подглазничной области. Последующее исследование показало, что положительные результаты наблюдались у тех, кто был ближе всего к желаемой массе тела [10–13].
Позже стал прицельно исследоваться механизм действия ФХ как активный компонент для разрушения жировых клеток. Основные предполагаемые механизмы включили вызываемый ФХ апоптоз клеток и активацию гормончувствительной липазы [14–16]. Путем взятия биоптатов жировой ткани до и после лечения ФХ, который был солюбилизирован ДХ, установили, что клеточные стенки были разрушены, а также возник воспалительный процесс, что привело к образованию рубцовой ткани [10].
Несмотря на исследование, проведенное в 2004 г., которое показало, что ДХ лизирует в культуре клеток человека кератиноциты, жировые и мышечные клетки, ученые не спешили признавать его ключевую роль в лизисе клеток при инъекционном липолизе [10, 17–19]. Отчасти это было связано с нерастворимостью ФХ в воде, что не позволяло проводить его безопасную оценку в моновиде в клинических исследованиях при участии добровольцев [12].
Решающее исследование, посвященное разногласиям по поводу главного активного компонента в липолитических растворах, проведено в 2010 г. ФХ растворяли в инертном минеральном масле, а цитотоксичность в отношении культивируемых адипоцитов измеряли с использованием окрашивания масляным красным О и измерением уровня лактатдегидрогеназы. Это исследование убедительно продемонстрировало, что ДХ является активным агентом и что ФХ сам по себе не вызывает лизиса клеток [16, 20].
Однако ФХ играет важную роль в комбинированном препарате. Он способен снижать интенсивность и тяжесть некроза жировой клетчатки, а также уменьшать образование рубцов [20, 21]. Хотя механизм, с помощью которого ФХ проявляет эти свойства, окончательно не определен.
В настоящее время существует несколько гипотез: ФХ действует как буфер для ДХ, поскольку pH раствора ДХ-ФХ ближе к pH ткани человека, чем pH раствора ДХ в отдельности, ФХ, растворенный в желчной соли дезоксихолата, использовался в качестве внутривенного лекарства для предотвращения или лечения жировой эмболии [21].
Исходя из этих данных, рекомендуется использовать ДХ при небольших локализованных жировых отложениях, а комбинацию ФХ и ДХ применять для более объемного вмешательства [16]. Наблюдение, что постинъекционное разрешение воспаления происходит быстрее при применении комбинации ФХ и ДХ по сравнению с применением только ДХ, подтверждает эти рекомендации [22].
Дезоксихолевая кислота относится к группе вторичных желчных кислот, которые синтезируются микрофлорой толстого кишечника из первичных желчных кислот (например холевой, которая в свою очередь образуется в печени из холестерина), 90–95% ДХ кислоты реабсорбируется в кишечнике и поступает в кровь, затем вновь секретируется печенью в просвет кишечника в составе желчи. ДХ разрушает клеточную стенку адипоцитов, что приводит к излитию содержимого в межклеточное пространство, опосредованному воспалительному некрозу клеток и уменьшению числа адипоцитов. В отличие от ФХ ДХ является водорастворимым соединением. В качестве экзогенного химического вещества ДХ действует как мягкий детергент для солюбилизации ФХ. С химической точки зрения ДХ может существовать в четырех формах – мицеллы, везикулы, мономера и кристалла. ДХ в мономерной или кристаллической форме приводит к повреждению клеток, но в виде мицелл мобилизует жиры, высвобождаемые из адипоцитов. Интересно, что при инъекционном липолизе форма, в которой существует ДХ, в основном зависит от его концентрации.
В нескольких исследованиях in vivo и in vitro предприняты попытки более детального изучения механизма действия ДХ при инъекционном липолизе. Одно из исследований демонстрирует, что ДХ является активным компонентом, вызывающим лизис и гибель клеток [16]. Другое исследование показало, что инъекции дезоксихолата в липомы приводили к значительному уменьшению их размера. Эти эффекты подтверждены ультразвуковым исследованием. Было замечено, что при повышении концентрации ДХ оказывал неблагоприятное действие на липомы, но оно ограничивалось местными реакциями (длительное жжение и болезненность) [22–24].
Исследования на адипоцитах человека, обработанных увеличивающимися концентрациями ДХ, продемонстрировали, что его применение способно приводить к обширному лизису жировых клеток. Авторы отмечают, что комбинация ДХ с ФХ привела к значительному снижению гибели клеток, вызываемой ДХ в моновиде, что позволяет предположить опосредованный ФХ защитный эффект, способный снижать агрессивность воздействия ДХ [20, 21]. Эти результаты подтверждены гистологическими исследованиями с использованием серии биопсий жировой ткани человека. Дополнительные эксперименты с клеточными культурами показали, что ДХ лизирует не только жировые клетки, но и фибробласты, эндотелиальные клетки и миоциты [21, 24, 25], что говорит об универсальном характере его действия по отношению к мембранам вне зависимости от типа клеток.
Показания и противопоказания к проведению инъекционного липолиза
Основные показания к инъекционному липолизу: коррекция целлюлита, локальная коррекция избыточной жировой ткани, нарушение микроциркуляции и лимфатический застой подкожно-жировой клетчатки отдельно взятых анатомических областей (за счет сокращения объема жировой ткани и последующего воспалительного каскада происходит усиление дренажной функции), решение эстетических задач по улучшению контуров тела на различных участках.
Основные противопоказания к проведению инъекционного липолиза: беременность и период лактации, онкологические заболевания, отягощенный аллергоанамнез, сахарный диабет, заболевания печени, сердечно-сосудистые заболевания, аутоиммунные, инфекционные заболевания, ожирение (индекс массы тела >30), возраст до 18 лет и пожилой возраст старше 55 лет [26, 27].
Топография применения инъекционного липолиза
Анатомические контуры тела с основными участками скопления жировых отложений индивидуальны для каждого человека, тем не менее выделяют общие зональные точки воздействия, где допустимо проведение данной процедуры: область внутренней поверхности плеч, надлоктевая складка, локальные жировые отложения в области передней брюшной стенки, жировые складки туловища, спины, жировые отложения в зоне VII шейного позвонка («вдовий горб»), жировые пакеты в области нижней трети лица, подбородка и шеи [28].
Согласно нашим клиническим наблюдениям (неопубликованные данные), с особой осторожностью следует проводить инъекции липолитиков, содержащих ФХ/ДХ, в области коленных суставов из-за риска демиелинизации чувствительных нервных волокон и длительной потери локальной чувствительности. Возможность повреждения нервов при данной процедуре описана в клинических исследованиях и наблюдениях.
H.M. El-Gowelli et al. изучили локальное влияние повторных инъекций ФХ/ДХ на скелетные мышцы и нервную ткань. С этой целью самкам крыс осуществляли чрескожное введение липолитика в течение 4 дней подряд (контроль – физиологический раствор). Гистопатологическое исследование показало, что раствор ФХ/ДХ вызывал повреждение нервной ткани и приволил к выраженному воспалению в месте инъекции, результатом которого стали дегенерация, некроз и фиброз скелетных мышц. Электронномикроскопическое исследование нервных волокон в области инъекции показало наличие интранейральных фибробластов, отложение интранейральных коллагеновых волокон и выраженную дегенерацию миелина. Помимо этого введение ФХ/ДХ привело к утолщению стенок интранейральных кровеносных сосудов и появлению эндоневральных тучных клеток. Результаты исследования подчеркивают возможный риск нервно-мышечного повреждения, связанный с повторными инъекциями ФХ/ДХ в процессе коррекции нежелательных жировых отложений и липом [29].
A.D. Blandford et al. [30] доказали возможность прямой нейротоксичности ДХ. Исследование проводили на свежем трупном материале. Производился забор сегмента краевой нижнечелюстной ветви лицевого нерва. Его подвергали воздействию ДХ (10 мг/мл) в течение 20 минут и ДХ (10 мг/мл) в течение 24 часов с последующим гистологическим исследованием. Контроль – физиологический раствор. Интенсивность окрашивания срезов толуидиновым синим оценивали с помощью световой микроскопии и анализа изображения с деконволюцией цвета. Результаты показали, что ДХ привел к повреждению миелиновой оболочки. Авторы пришли к выводу: прямая нейротоксичность ДХ может вызывать клиническое значимое повреждение нерва.
Изучение влияния ДХ на краевую нижнечелюстную ветвь лицевого нерва выбрано авторами не случайно, т.к. в литературе описаны неоднократные случаи его повреждения вследствие инъекции липолитиков, содержащих в своем составе ДХ. Подобная нейропраксия приводит к временному дисбалансу мимических движений, а также к слюнотечению из-за неполного смыкания ротовой щели [31–33].
Побочные эффекты и осложнения
Местные реакции:
- к ожидаемым реакциям, наблюдаемым сразу после процедуры, относятся отек, эритема, гематомы, зуд и жжение (последние проявления связаны с гистаминолибераторной активностью ФХ) [34–36]. В течение первых суток у большинства пациентов развивается умеренно-выраженная болезненность в области инъекций, усиливающаяся при движении (следствие локального воспаления в подкожно-жировой клетчатке), купируется самостоятельно;
- местные аллергические реакции (крапивница) [37];
- единичные случаи стойкого ощущения зуда или жжения (обусловлены гиперчувствительностью к компонентам инъекционной смеси, чаще всего к консерванту – бензиловому спирту) [38];
- нейропраксия [30, 31];
- транзиторная гиперпигментация и персистирующая болезненность (от 2 недель до 3 месяцев) [39].
К общим реакциям относятся:
- анафилактические реакции [37, 40];
- холестаз печени и внутрисосудистый гемолиз (к этому может приводить один из продуктов деградации ФХ – лизофосфахатидилхолин) [4, 40];
- системная воспалительная реакция, распространенный септальный панникулит [41–43];
- абдоминальная боль, тошнота, рвота, гиперсаливация при разовом применении ФХ в дозировке, превышающей 2,5 г [35].
Клинический случай
В отделение дерматологии и косметологии АО «Институт пластической хирургии и косметологии» обратилась пациентка А. 48 лет с жалобой на множественные участки уплотнения кожи и отек нижней трети лица и подподбородочной области, развившиеся после инъекции липолитиков (рис. 1).
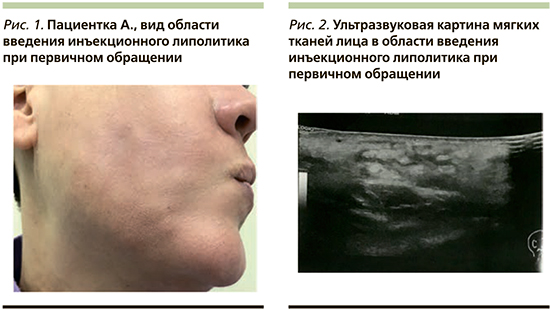
Из анамнеза: пациентке проведено 4 процедуры инъекционной липолитической терапии в область нижней трети лица и подподбородочную область с интервалом 10 дней. На следующий день после 4-й процедуры пациентка отметила появление множественных уплотнений в области инъекций, покраснение кожи и ощущение жара. Она самостоятельно пропила курс антибиотика (амоксициллин+клавулановая кислота). Без эффекта.
Со слов пациентки применялся липолитический «коктейль», в состав которого входили следующие компоненты (записаны ею со слов врача, проводившего процедуру): гиалуроновая кислота, никотинамид, DMAE (диметиламиноэтанол), ДХ. При более подробном сборе анамнеза выяснено, что данный липолитик несертифицирован в РФ.
При обращении к врачу, проводившему процедуру, назначена терапия: мезотерапевтический препарат с PDRN (полидезоксирибонуклеотиды), 2 процедуры без эффекта, микротоковая терапия и дарсонваль без эффекта, внутриочаговые инъекции комплексным ферментным препаратом с коллагеназой и гиалуронидазой (несертифицирован в РФ) без эффекта.
Хронические заболевания: рассеянный склероз. Пациентка проходит лечение препаратом интерферона β-1b. Аллергический анамнез не отягощен.
Объективно: кожа в области нижней трети лица и подподбородочной области отечна. Цвет кожи неярко выраженного цианотичного оттенка, общая и местная температура в пределах нормы. При пальпации отмечаются неравномерные множественные шаровидные участки каменистой плотности с минимальной подвижностью, болезненности не наблюдается.
Заключение ультразвукового исследования: в проекции пальпируемых уплотнений лица в подподбородочной области и нижней трети обеих щечных областей определяются УЗ-признаки выраженного отека с включениями межтканевой жидкости на глубине от 5 мм, изменения локализуются преимущественно вокруг мест инъекции липолитического препарата. Сосудистый рисунок при цветовом допплеровском картировании не усилен, достоверно инфильтративно-полостных структур не выявлено. Слюнные железы интактны (рис. 2). От проведения биопсии пациентка отказалась.
Диагноз по Международной классификации болезней: T81.8. Другие осложнения процедур, не классифицированные в других рубриках. Клинический диагноз: постинъекционная воспалительная реакция кожи и мягких тканей лица.
Пациентке проведена медикаментозная терапия: дексаметазон перорально по схеме: 1-е сутки – 9 мг, 2-е – 6 мг, 3-и – 3 мг, 4-е – 1,5 мг, 5-е сутки – 0,75 мг. При снижении дозировки до 0,75 мг и в течение последующих двух суток пациентка отметила рецидив отека и уплотнений слабой степени выраженности, вследствие чего назначена поддерживающая терапия дексаметазоном 2 мг/сут в течение 2 недель. Дополнительные назначения: аспаркам 2 таблетки в сутки, омепразол 20 мг/сут 2 недели.
Физиотерапия: ультразвук с дексаметазоном № 10+фармафорез с лонгидазой № 10 (с чередованием каждый день). Наружная терапия – крем с гиалуронидазой 2 раза в сутки (3 недели).
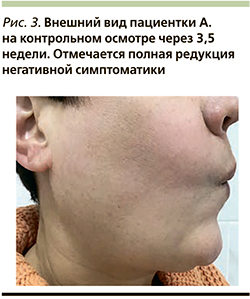
Через 3,5 недели на контрольном осмотре отмечена полная редукция негативной симптоматики (рис. 3). Пациентка находилась под динамическим наблюдением в течение 1,5 лет. Рецидива воспалительной реакции не отмечено.
Обсуждение
Несмотря на многолетний мировой опыт применения инъекционных липолитиков, описания побочных эффектов, подобных приведенному нами, в научной литературе встречаются лишь эпизодически.
Z. Kutlubay et al. [44] опубликовали клинический случай формирования гранулем инородного тела, возникших после инъекционной липотерапии: пациентка обратилась с жалобой на очаги уплотнений в области задней поверхности бедер, возникшие после введения липолитика и сохранявшиеся на протяжении 1,5 лет.
Сеансы липотерапии (состав, содержащий ФХ/ДХ, карнитин, буфломедил, прокаин, физиологический раствор) проводились 1 раз в 2 недели. Через 3 дня после третьей процедуры в местах введения препарата появились небольшие отечные эритематозные папулы, которые постепенно прогрессировали до образования узелков. В связи с этим были назначены местные и системные антибиотики в течение месяца (подозревалась вторичная бактериальная инфекция) – без эффекта. Гистопатологическое исследование выявило наличие палисадно расположенных эпителиоидных гистиоцитов и многоядерных гигантских клеток. Микробиологические исследования были отрицательными. Пациентке проведена ежемесячная терапия внутриочаговыми инъекциями триамцинолона (40 мг/мл), всего 3 процедуры. Через 3 месяца наблюдался выраженный регресс узелков и папул, однако завершившийся атрофией кожи.
Клинический случай, приведенный нами в статье, имел схожую клиническую картину. Высоковероятным следствием данного воспалительного процесса могло стать формирование множественных гранулем, но благодаря раннему обращению пациентки за медицинской помощью и своевременно оказанной терапии нам удалось это предотвратить.
Заключение
Несмотря на то что инъекционный липолиз уже много лет широко применяется в общемировой косметологической практике, протоколы проведения процедуры по-прежнему далеки от стандартизации, а перечень возможных побочных эффектов до конца не установлен. Ограниченное число достоверных клинических исследований безопасности и эффективности липолитиков в основном посвящены изучению таких ингредиентов, как ФХ/ДХ. Но помимо этих веществ на рынке присутствует множество разнообразных ингредиентов, рецептур и инъекционных «коктейлей» с липолитическим эффектом, однако лишь единицы из них являются сертифицированными препаратами, а тенденция к снижению доли некачественных препаратов отсутствует.
Большинство пациентов, а в некоторых случаях и сами врачи склонны недооценивать многочисленные потенциальные побочные эффекты, которые могут возникать при использовании данного вида инъекционной терапии. Чтобы свести к минимуму риск побочных эффектов, необходима стандартизация этой процедуры и более детальное изучение эффективности и безопасности химических соединений, входящих в состав липолитиков. Немаловажна необходимость правильно диагностировать и лечить пациентов с развившимися осложнениями вследствие липотерапии. Следует подчеркнуть, что инъекционный липолиз не является заменой хирургической липосакции. Однако его применение для коррекции ограниченного объема жировой ткани или при наличии противопоказаний к оперативному вмешательству нередко позволяет достигать выраженного эстетического результата.



