Введение
В настоящее время с развитием персонифицированного подхода к медицине при выборе терапии все более широкое применение получают препараты моноклональных антител (МАТ), использующиеся для лечения заболеваний, поражающих различные органы и системы, в т.ч. для лечения различных типов рака, ревматоидного артрита, бронхиальной астмы, рассеянного склероза (РС).
Препараты МАТ были одобрены для лечения более чем 30 нозологий. МАТ обладают исключительной избирательностью к терапевтической мишени, следовательно, меньшей токсичностью. Число пациентов, получающих препараты данной группы, ежегодно увеличивается.
Препараты МАТ являются одними из наиболее предпочтительных вариантов лечения РС — хронического иммуноопосредованного заболевания центральной нервной системы. Это связано с их высокой эффективностью и специфичностью к отдельным компонентам иммунной системы, играющим важную роль в патогенезе данного заболевания [1].
Одним из часто используемых препаратов для лечения РС является алемтузумаб. Препарат продемонстрировал эффективность в отношении снижения частоты рецидивов и прогрессирования инвалидности как среди пациентов с РС, ранее получавших терапию интерферонами в, так и среди нелеченых пациентов [2, 3]. С учетом доказанной высокой эффективности алемтузумаб одобрен для лечения рецидивирующеремиттирующего РС у взрослых более чем 50 стран, в т.ч. России. Терапия проводится за два курса: во время первого курса препарат вводится внутривенно в дозе 12 мг/сут в течение 5 последовательных дней. Второй курс проводится через 12 месяцев после первого и подразумевает введение препарата в той же дозе в течение 3 последовательных дней.
Алемтузумаб представляет собой первый лекарственный препарат гуманизированного человеческого антитела, связывающийся с мембранными гликопротеинами CD52 В- и Т-лимфоцитов, моноцитов и макрофагов, что приводит к их лизису, длительному истощению пула и последующей репопуляции [4]. Острый иммуносупресивный эффект алемтозумаба сопровождается дальнейшим восстановлением иммунных клеток. Обычно вначале восстанавливаются В-лимфоциты и моноциты, затем CD3+- и CD4+- Т-лимфоциты. Репопуляция сопровождается изменением в подгруппах лимфоцитов, в частности увеличением уровня регуляторных Т-лимфоцитов, а также В- и Т-лимфоцитов памяти. Вышеописанные процессы вызывают перестройку иммунной системы: более быстрое восстановление В-клеток без адекватного регуляторного контроля со стороны Т-клеток может лежать в основе активации аутоиммунных процессов на фоне терапии алемтузумабом [5]. Также терапия алемтузумабом может приводить к образованию аутоантител и повышать риск развития иммуноопосредованных побочных эффектов, среди которых наиболее часто наблюдаются аутоиммунные заболевания щитовидной железы (ЩЖ) [6].
Заболевания ЩЖ в популяции
Аутоиммунные заболевания ЩЖ — одни из наиболее часто встречающихся аутоиммунных заболеваний в популяции, их распространенность может достигать 5% [7]. Риск развития аутоиммунного заболевания ЩЖ увеличивается при уже имеющемся аутоиммунном заболевании, в т.ч. при РС [8].
Аутоиммунные заболевания ЩЖ чаще всего представляют собой три основных варианта: аутоиммунный гипотиреоз, болезнь Грейвса и безболевой тиреоидит, однако встречаются и более редкие формы.
Аутоиммунный гипотиреоз является вариантом хронического лимфоцитарного тиреоидита, чаще всего приводящего к необратимому гипотиреозу вследствие разрушения ткани ЩЖ. Лечение гипотиреоза заключается в приеме синтетического тироксина.
Болезнь Грейвса представляет собой второй по распространенности вариант аутоиммунных тиреопатий и проявляется тиреотоксикозом, вызванным стимуляцией рецепторов тиреотропного гормона (ТТГ) посредством антител к данному рецептору (АТ к рТТГ). В некоторых случаях в организме человека вырабатываются нейтральные или блокирующие антитела к рецептору ТТГ. Лечение болезни Грейвса может быть консервативным: прием антитиреоидных препаратов, блокирующих синтез гормонов ЩЖ на протяжении 12—18 месяцев; или радикальным: тиреоидэктомия или терапия радиоактивным йодом. Метод лечения выбирается клиницистом в соответствии с особенностями течения заболевания, наличием или отсутствием осложнений и сопутствующей патологии, а также с учетом пожеланий пациента.
Третьим вариантом аутоиммунной патологии ЩЖ является безболевой тиреоидит, имеющий волнообразное течение. На начальном этапе заболевания развивается тиреотоксикоз, что связано с высвобождением уже синтезированных гормонов из поврежденной ЩЖ. В дальнейшем по мере элиминации тиреодных гормонов из организма тиреотоксикоз сменяется эутиреозом, затем - гипотиреозом. Со временем функция ЩЖ обычно полностью восстанавливается.
Другим воспалительным заболеванием ЩЖ является подострый тиреоидит, или тиреоидит Де Кервена. Это заболевание предположительно вирусной этиологии, сопровождающееся деструктивным тиреотоксикозом и болевым синдромом в области шеи. В качестве терапии данного заболевания применяются нестероидные противовоспалительные средства и глюкокортикоиды. По своему течению подострый тиреоидит сходен с безболевым: для него также характерно чередование преходящих во времени тиреотоксической и гипотиреоидной фаз.
Еще одной причиной развития синдрома тиреотоксикоза может быть автономная гиперпродукция тиреодных гормонов узловыми образованиями ЩЖ. Причиной возникновения данной патологии служит длительная стимуляция ЩЖ на фоне структурной гетерогенности тироцитов в условиях йодного дефицита. Предпочтителен радикальный вариант лечения: терапия радиоактивным йодом или тиреоидэктомия, т.к. применение тиреостатиков даст лишь временный эффект и их отмена приведет к возобновлению тиреотоксикоза.
Заболевания ЩЖ, индуцированные алемтузумабом
Аутоимунные заболевания ЩЖ являются наиболее частым иммуноопосредованным побочным эффектом у пациентов с РС, получающих терапию алемтузумабом. Они могут развиваться через 6 месяцев после начала терапии и достигать пика заболеваемости через 3 года с последующим постепенным снижением.
В исследованиях 3-й фазы у 40,7% (CARE-MS I) и 37,7% (CARE-MS II) пациентов, получавших терапию алемтузумабом, иммуноопосредованная патология ЩЖ развивалась в течение 5 лет от старта терапии и пик заболеваемости пришелся на третий год от начала лечения [9-11].
В отличие от общей популяции аутоимунные заболевания ЩЖ у пациентов с РС после терапии алемтузумабом чаще проявляются тиреотоксикозом, в частности болезнью Грейвса, предполагаемая распространенность которой составляет от 16,7 до 41% [12].
Важно отметить, что подобная высокая распространенность болезни Грейвса не отмечалась среди пациентов, получавших алемтузумаб по поводу ревматоидного артрита или после трансплантации органов. Этот факт позволяет предположить, что пациенты с РС более подвержены риску развития алемтузумаб-индуцированных тиреопатий, в частности болезни Грейвса. С клинической точки зрения болезнь Грейвса, развившаяся после терапии алемтузумабом, характеризуется удивительно высокой частотой ремиссии, как спонтанной, так и на фоне терапии тиреостатиками, а также переходом в гипотиреоз, который, вероятнее всего, служит следствием трансформации стимулирующих АТ к рТТГ в блокирующие.
Другой особенностью алемтузумабиндуцированных тиреопатий является высокая распространенность гипотиреоза, вызванного действием блокирующих АТ к рТТГ (до 50% всех случаев) [13].
Таким образом, аутоимунные тиреопатии, вызванные алемтузумабом, имеют неклассические клинические проявления и могут представлять определенные сложности при диагностике и ведении. В настоящее время в соответствии с рекомендациями Европейской тиреодологической ассоциации пациентам после терапии алемтузумабом целесообразно проводить контроль уровня ТТГ каждые 3 месяца для своевременного выявления и коррекции нарушений функции ЩЖ [13].
Клиническое наблюдение
Пациентка Л. 59 лет поступила в эндокринологическое отделение в сентябре 2019 г. с жалобами на слабость, утомляемость, учащенное сердцебиение, потливость, осиплость голоса, затруднения глотания, болезненность в области передней поверхности шеи, повышение температуры до 38,4°С. Вышеуказанные жалобы возникли в августе 2019 г.
Из анамнеза известно, что в 2006 г. пациентке установлен диагноз «рассеянный склероз», в 2016 и 2017 гг. получала терапию алемтузумабом. В 2010 г. на фоне терапии препаратами интерферона у пациентки развился эпизод цитокин-индуцированного тиреотоксикоза, по поводу которого была назначена терапия преднизолоном.
При контроле ТТГ каждые 3 месяца в течение 2 лет после лечения у пациентки сохранялся эутиреоз (рис. 1).
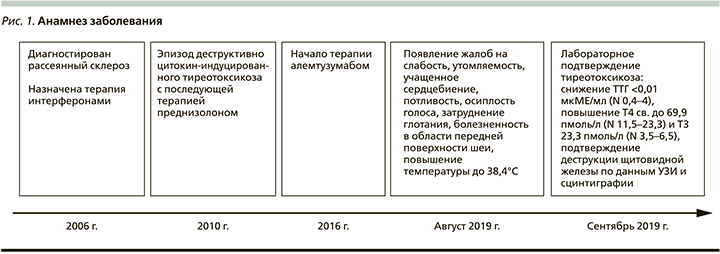
При обследовании в отделении был выявлен тиреотоксикоз: снижение уровня ТТГ менее 0,01 мкМЕ/мл (0,4-4), повышение Т3 до 23,3 пмоль/л (3,5-6,5), Т4 до 69,9 пмоль/л (11,523,2), АТ-ТГ более 2500 МЕ/мл (0-60), АТ-ТПО - 66 МЕ/мл (0-60), уровень АТ к рецептору ТТГ составил менее 0,8 МЕ/л (0-9,999).
По данным общего анализа крови обращало на себя внимание повышение скорости оседания эритроцитов (СОЭ) по Вестергрену до 69 мм/ч при нормальном уровне лейкоцитов 8,6х109/л.
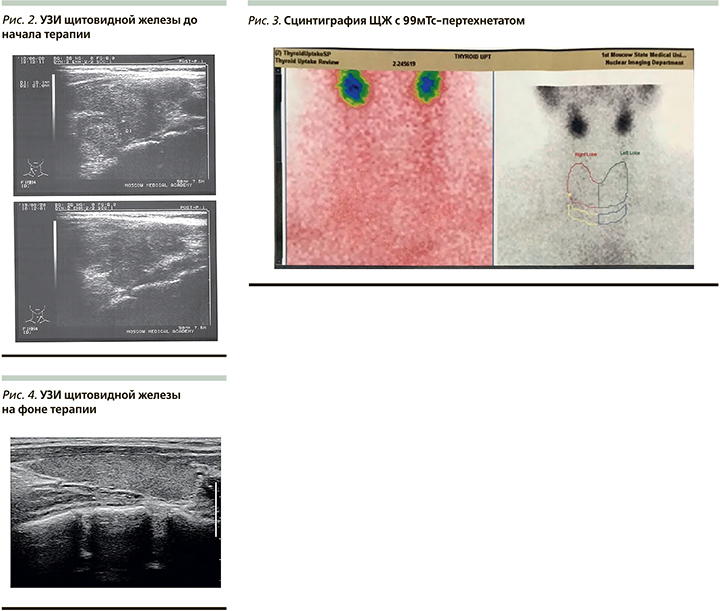
По данным ультразвукового исследования (УЗИ) ЩЖ, выявлено увеличение общего объема ЩЖ до 32,5 мл и узловое образование в нижней трети правой доли - солидный узел средней эхогенности овоидной формы с ровными контурами, 1,6*1,9x2,0 см (3-я категория образований по классифакции EU - TIRADS), а также диффузные изменения паренхимы ЩЖ, характерные для подострого тиреоидита (рис. 2)
Для верификации причины тиреотоксикоза пациентке проведена сцинтиграфия с 99мТс-пертехнетатом. По результатам сцинтиграфии ЩЖ с пертехнетатом выявлено тотальное снижение общей накопительной функции ЩЖ, индекс захвата составил 0,5% (1-1,8), что характерно для деструктивного процесса в ткани ЩЖ. Признаков функционально автономных образований не выявлено (рис. 3).
Принимая во внимание клиническую картину: наличие болезненности в области передней поверхности шеи, подъемы температуры тела, повышение СОЭ, ультразвуковые признаки, характерные для подострого тиреоидита, для дифференциальной диагностики с алемтозумаб-индуцированным тиреоидитом пациентке был проведен тест Крайля - назначен преднизолон в дозе 30 мг/сут. На фоне проводимой терапии глюкокортикоидами пациентка отметила улучшение самочувствия: нормализацию температуры тела, уменьшение болезненности в области передней поверхности шеи.
По данным лабораторного контроля, на фоне 8 дней терапии преднизолоном выявлено снижение СОЭ по Вестергрену до 28 мм/ч, уровни св. Т3 и св. Т4 соответствовали референсным значениям (4,2 и 22,0 пмоль/л соответственно). Данные представлены в таблице.
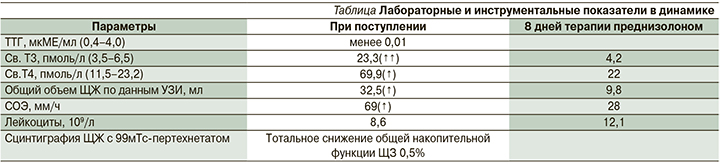
При УЗИ ЩЖ на фоне терапии преднизолоном в дозе 30 мг/сут. в течение 6 дней отмечалось уменьшение общего объема ЩЖ с 32,5 до 9,8 мл и уменьшение участков воспаления в левой доле (рис. 4).
Обсуждение
У пациентки спустя 3 года после терапии алемтузумабом развилась клиническая картина тиреотоксикоза, что соответствует срокам пиковой манифестации патологии ЩЖ у пациентов на данной терапии. С учетом наибольшей распространенности среди алемтузумаб-индуцированных тиреопатий у пациентки можно было заподозрить манифестацию болезни Грейвса. В то же время наличие в анамнезе эпизода деструктивного тиреоидита указывало на высокий риск его повторного развития.
Учтя клиническую картину: наличие болезненности в области передней поверхности шеи, подъемы температуры тела, повышение СОЭ, ультразвуковые признаки, у пациентки был заподозрен подострый тиреоидит - маловероятное и нетипичное состояние на фоне терапии алемтузумабом. Также наличие узлового образования ЩЖ, по данным УЗИ, не позволяло исключить у пациентки функциональной автономии.
Для верификации причины тиреотоксикоза проведены сцинтиграфия ЩЖ и тест Крайля.
Учтя полученные клинико-лабораторные данные, низкий индекс захвата по данным сцинтиграфии, положительный тест с преднизолоном, а также уменьшение общего объема ЩЖ и размеров участков воспаления в левой доле по УЗИ, у пациентки диагностирован подострый тиреоидит.
Однако это не исключает возможности развития иммуноопосредованных тиреопатий в последующем, в связи с чем чрезвычайно важно продолжить регулярный контроль функции ЩЖ на протяжении по меньшей мере 4 лет после терапии алемтозумабом.
Выводы
Этот случай неклассического варианта тиреотоксикоза на фоне лечения препаратами МАТ иллюстрирует необходимость регулярного контроля ТТГ у пациентов с РС после терапии алемтузумабом для своевременного выявления и коррекции дисфункции ЩЖ.


