Лечение мигрени завтра и послезавтра. Влияние на путь CGRP
DOI: https://dx.doi.org/10.18565/pharmateca.2019.13.58-62
К.В. Скоробогатых, Ю.Э. Азимова
Университетская клиника головной боли, Москва, Россия
Мигрень – это хроническое неврологическое заболевание, характеризующееся высокой распространенностью и значительным снижением качества жизни. Сочетание этих факторов приводит к существенному негативному социоэкономическому влиянию. В последнее время происходит заметный прогресс в понимании патогенеза мигрени, что приводит к появлению новых классов препаратов для лечения этого заболевания. В обзоре рассмотрены история изучения кальцитонин-ген родственного пептида (CGRP) в патогенезе мигрени и характеристики новых препаратов, воздействующих на путь CGRP-антагонистов CGRP-рецепторов («джепантов») и моноклональных антител к рецептору CGRP/CGRP.
Ключевые слова: мигрень, CGRP, кальцитонин-ген родственный пептид, джепанты, моноклональные антитела
Введение
Мигрень – это хроническое неврологическое заболевание. Оно входит в тройку самых распространенных в мире и является самым часто встречаемым неврологическим заболеванием [1]. Распространенность мигрени в мире затрагивает около 15% населения, в России – более 20% [2]. Помимо высокой распространенности мигрень оказывает значительное снижение качества жизни пациентов: по показателю YLD (Years Lived with Disability, число лет, прожитых с нетрудоспособностью) мигрень находится на седьмом месте в мире среди всех известных заболеваний [3]. Сочетание высокой распространенности и значительного снижения качества жизни приводит к значительным экономическим затратам – более 111 млрд евро ежегодно в Европейском Союзе [4] и около 23 млрд долл. США в России [5]. Сочетание этих фактов делает поиск эффективных и безопасных методов лечения крайне актуальной проблемой.
Основные подходы к лечению мигрени: изменение образа жизни, лечение (купирование) приступа мигрени и профилактическое лечение (предотвращение возникновения приступов). Для купирования приступов в настоящее время применяются нестероидные противовоспалительные средства, триптаны, эрготаминсодержащие средства, противорвотные препараты [6, 7], некоторые методы нейростимуляции. Основу профилактического лечения мигрени до недавнего времени составляли β-адреноблокаторы, антидепрессанты, противосудорожные, ингибиторы ангиотензинпревращающего фермента, блокаторы рецептора АТ2, ботулинический токсин типа А [8]. Как видно из этого списка, большинство препаратов для лечения мигрени неспецифические и не были разработаны непосредственно для ее лечения. Эта ситуация связана в первую очередь с тем, что патогенез мигрени до конца не изучен [9]. Однако в последнее время произошел значительный прогресс в понимании этого вопроса, что привело к появлению совершенно новых групп препаратов для лечения мигрени.
Нейробиология мигрени
Патофизиология мигрени представляет весьма сложное сочетание изменения активности различных зон головного мозга (коры, гипоталамуса, моста), изменений концентрации нейромедиаторов, а также генетической предрасположенности [9]. За последние 50 лет произошел сдвиг от «сосудистой теории» мигрени [10], предложенной еще в 1940-х гг., к пониманию мигрени как неврологического заболевания. В 1979 г. М. Московиц впервые показал анатомическую связь между волокнами тройничного нерва и сосудами твердой мозговой оболочки и предложил тригемино-васкулярную (или нейроваскулярную) теорию мигрени [11]. В 1985 г. Л. Эдвинссон определил, что одну из ключевых ролей в работе этой системы играет CGRP (calcitonin-gene related peptide, кальцитонин-ген родственный пептид) [12]. Этот пептид представляет собой молекулу из 37 аминокислот и образуется в результате альтернативного сплайсинга гена, кодирующего кальцитонин, содержится в центральной и периферической нервных системах, а также является мощным вазодилататором [13]. В дальнейшем в серии экспериментов Л. Эдвинссон и П. Годсби показали, что концентрация белка CGRP в крови повышается при стимуляции ганглия тройничного нерва у кошек и человека [14], при стимуляции верхнего сагиттального синуса у кошек по сравнению с уровнем до стимуляции [15]. Эти данные показывают, что CGRP играет одну из ключевых ролей в тригемино-васкулярной системе, выделяясь при стимуляции как ее «невральной», так и «сосудистой» части. Кроме того, П. Годсби и Л. Эдвинссон продемонстрировали, что у пациентов с мигренью уровень CGRP в крови, взятой из яремной вены, значительно выше в момент приступа мигрени по сравнению с концентрацией в межприступный период [16]. Ряд последующих работ показал влияние суматриптана на падение концентрации CGRP одновременно с регрессом приступа мигрени [17] и то, что введение CGRP провоцирует типичный для пациентов приступ мигрени [18]. CGRP достаточно широко распространен в центральной и периферической нервных системах (менингеальная оболочка, ганглий тройничного нерва, ядро спинномозгового пути тройничного нерва, мост, гипоталамус, мозжечок) [19]. Л. Эдвинссон показал, что в ганглии тройничного нерва находятся небольшие нейроны, которые содержат преимущественно CGRP, и более крупные нейроны, содержащие рецептор к CGRP. Подобная ситуация сохраняется и в области твердой мозговой оболочки, где рецептор и лиганд находятся в различных волокнах тройничного нерва [20]. Эти данные позволяют предполагать, что в CGRP-опосредованном пути развития приступа мигрени ключевые события происходят именно в этих зонах тригемино-васкулярной системы.
Антагонисты CGRP-рецепторов («джепанты»)
Эти и ряд других данных привели к пониманию того, что воздействие на путь CGRP может быть использовано для терапии мигрени. В начале 2000-х гг. появился новый класс препаратов – антагонисты CGRP-рецепторов, или «джепанты». Первым из этой группы препаратов был олцеджепант. Датский невролог J. Olesen показал его преимущество над плацебо для купирования приступа мигрени по показателю устранения головной боли через 2 часа [21]. Олцеджепант был разработан для внутривенного введения как подтверждение концепции эффективности джепантов и не предполагался к клиническому применению. Второй препарат этой группы, телкаджепант, был разработан для перорального приема и также был эффективнее, чем плацебо, для купирования приступов мигрени [22]. К сожалению, в исследованиях телкаджепанта для профилактики мигрени была выявлена гепатотоксичность [23] и все исследования этого препарата и других джепантов были на некоторое время прекращены. Но через некоторое время стало ясно, что этот побочный эффект не стал класс-специфичным, а присущ только одному телкаджепанту, и исследования других молекул были продолжены. В настоящее время успешно закончились клинические исследования уброджепанта и римеджепанта для купирования приступа мигрени. Препараты были эффективнее плацебо как по показателю устранения боли через два часа, так и по устранению сопутствующих симптомов (тошноты, фоно/фотофобии). Побочных эффектов, связанных с повреждением печени, в исследованиях выявлено не было [24–26]. Также успешно закончилась 2b/3 фаза клинического исследования атоджепанта для профилактики мигрени [27]. Поскольку CGRP является мощным вазодилататором, особое внимание было уделено кардиоваскулярной безопасности. Ни в одном из исследований джепантов не было получено побочных эффектов, связанных с сердечно-сосудистой системой [24–27].
Моноклональные антитела к рецептору CGRP/белку CGRP
Помимо обычных препаратов («маленьких молекул») для влияния на путь CGRP были разработаны моноклональные антитела к рецептору CGRP и самому белку CGRP. Моноклональные антитела (МАТ) являются таргетной терапией и имеют ряд особенностей: большой период полувыведения, отсутствие метаболизма в печени, большой размер молекулы [28]. Их характеристики делают эти препараты максимально подходящими для профилактической терапии мигрени: редкий режим дозирования (1 раз в месяц или 1 раз в три месяца), сниженный риск межлекарственного взаимодействия, отсутствие проникновения через неповрежденный гематоэнцефалический барьер в существенных количествах [29]. В настоящее время разработано одно МАТ, блокирующее рецептор CGRP, – эренумаб, и три МАТ, блокирующих сам белок CGRP, – фреманезумаб, галканезумаб и эптинезумаб. Сводная характеристика этих молекул представлена в табл. 1 [30].
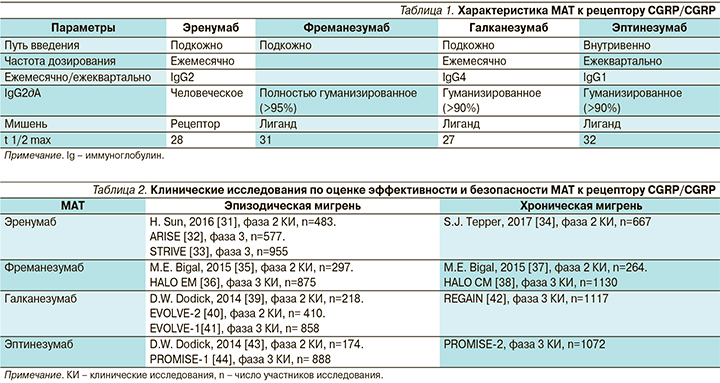
Все исследования фаз 2 и 3 всех четырех препаратов были положительными как при эпизодической, так и при хронической мигрени, и 3 из них (эренумаб, фреманезумаб и галканезумаб) уже доступны для клинического применения в ряде стран. Клинические исследования по оценке эффективности и безопасности МАТ к рецептору CGRP/CGRP суммированы в табл. 2.
Среди побочных эффектов, отмеченных в ходе клинических исследований, наиболее частыми были боль в месте инъекции и инфекции верхних дыхательных путей. Число этих побочных эффектов не различалось между группами активного препарата и группой плацебо ни в одном из опубликованных исследований МАТ к рецептору CGRP/CGRP. Число серьезных побочных эффектов не различалось между группами активного препарата и плацебо. Также незначительным было число анти-МАТ-антител и нейтрализующих антител, при этом наличие нейтрализующих антител не приводило к изменению эффективности или возникновению побочных эффектов [45].
Помимо исследований 2-й и 3-й фаз, показавших эффективность МАТ при эпизодической и хронической мигрени, были опубликованы результаты долгосрочных исследований безопасности и эффективности применения эренумаба в течение 3 лет [46] и фременезумаба в течение 12 месяцев [47]. В исследованиях было показано, что эффект препаратов сохраняется и даже имеет тенденцию к усилению на протяжении времени, а также не было выявлено дополнительных проблем с безопасностью.
Важным фактором для профилактической терапии мигрени является скорость наступления эффекта при назначении МАТ. В исследовании эренумаба и фреманезумаба различия между группами активного препарата и плацебо по числу дней с мигренью отмечались уже после первой недели после инъекции [48, 49]. В исследованиях эптинезумаба эффект проявлялся уже в первый день после введения препарата [50].
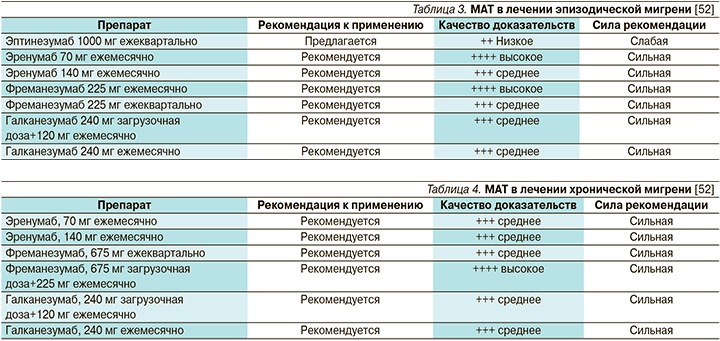
В 2019 г. Европейская федерация головной боли опубликовала клинические рекомендации по применению МАТ в лечении мигрени [52] (табл. 3, 4).
Заключение
Накопление научных данных о природе мигрени в последние 30 лет, в частности об участии CGRP в развитии приступа, позволило совершить качественный скачок в лечении этого заболевания: были разработаны и внедрены в клиническую практику новые препараты для профилактики мигрени – моноклональные антитела к рецептору CGRP/CGRP, успешно завершены клинические исследования антагонистов CGRP-рецепторов (джепантов). Но это лишь начало большого пути разработки новых классов специфических противомигренозных препаратов. В настоящее время успешно завершены или продолжаются исследования дитанов (агонистов 5HT-1F-рецепторов) для купирования приступа мигрени, а также моноклональных антител, влияющих на путь PACAP (Pituitary adenylatecyclase-activating peptide; пептид, активирующий аденилатциклазу гипофиза). Появляются новые методы неинвазивной нейромодуляции (стимуляция блуждающего нерва, тройничного нерва, транскраниальная магнитная стимуляция). Кроме этого выявляются новые мишени для терапии мигрени – орексины А и В и их рецепторы, АТФ-чувствительные калиевые и HCN-каналы [53].
Литература
1. Steiner T.J., et al. Migraine: the seventh disabler. J Headache Pain. 2013;14:1. Doi: 10.1186/1129-2377-14-1.
2. Ayzenberg I., Katsarava Z., Sborowski A., et al.; Lifting the Burden. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia. 2012;32(5):373–81. Doi: 10.1177/0333102412438977.
3. GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. Neurol. 2018;17(11):954–76. Doi: 10.1016/S1474- 4422(18)30322-3.
4. Linde M., Gustavsson A., Stovner L.J., et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2011;19(5):703–11. Doi: 10.1111/j.1468-1331.2011.03612.x.
5. Ayzenberg I., Katsarava Z., Sborowski A., et al. Headache-attributed burden and its impact on productivity and quality of life in Russia: structured healthcare for headache is urgently needed. Eur J Neurol. 2014;21(5):758–65. Doi: 10.1111/ene.12380.
6. Evers S., Afra J., Frese A., et al. European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine--revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81. Doi: 10.1111/j.1468-1331.2009.02748.x.
7. Marmura M.J., Silberstein S.D., Schwedt T.J. The Acute Treatment of Migraine in Adults: The American Headache Society Evidence Assessment of Migraine Pharmacotherapies. Headache. J Head Face Pain. 2015;55(1):3–20. Doi: 10.1111/head.12499.
8. Silberstein S.D. Preventive Migraine Treatment. Continuum (Minneap Minn). 2015;21(4 Headache):973–89. Doi: 10.1212/CON.0000000000000199.
9. Goadsby P.J., Holland P.R., Martins-Oliveira M., et al. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev. 2017;97(2):553–622. Doi: 10.1152/physrev.00034.2015.
10. Ray B., Wolff H. Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch Surg. 1940;41:813–56.
11. Moskowitz M.A., Reinhard J.F. Jr., Romero J., et al. Neurotransmitters and the fifth cranial nerve: is there a relation to the headache phase of migraine? Lancet. 1979;2(8148):883–85. Doi: 10.1016/s0140-6736(79)92692-8
12. Uddman R., Edvinsson L., Ekman R., et al. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci Letters. 1985;62(1):131–36. Doi: 10.1016/0304-3940(85)90296-4. Doi: 10.1016/0304-3940(85)90296-4.
13. Скоробогатых К.В., Табеева Г.Р. Кальцитонин-ген-родственный пептид в патогенезе первичных головных болей. Российский журнал боли. 2010;(1):1–52.
14. Goadsby P.J., Edvinsson L., Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988);23(2):193–96. Doi:10.1002/ana.410230214.
15. Zagami A.S., Goadsby P.J., Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropept. 1990;16(2):69–75. Doi: 10.1016/0143-4179(90)90114-e.
16. Goadsby P.J., Edvinsson L., Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–87. Doi: 10.1002/ana.410280213.
17. Goadsby P.J., Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993);33(1):48–56. Doi: 10.1002/ana.410330109.
18. Lassen L., Haderslev P., Jacobsen V., et al. CGRP May Play A Causative Role in Migraine. Cephalalgia. 2002;22(1):54–61. Doi: 10.1046/j.1468-2982.2002.00310.x.
19. Warfvinge K., Edvinsson L. Distribution of CGRP and CGRP receptor components in the rat brain. Cephalalgia. 2017. 033310241772887. Doi: 10.1177/0333102417728873.
20. Edvinsson L. The Trigeminovascular Pathway: Role of CGRP and CGRP Receptors in Migraine. Headache. J Head Face Pain. 2017;57:47–55. Doi:10.1111/head.13081.
21. Olesen J., Diener H.-C., Husstedt I. W., et al. Calcitonin Gene–Related Peptide Receptor Antagonist BIBN 4096 BS for the Acute Treatment of Migraine. N Engl J Med. 2004;350(11):1104–10. Doi: 10.1056/nejmoa030505.
22. Edvinsson L., Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376(9741):645–55. Doi: 10.1016/s0140-6736(10)60323-6.
23. Ho T.W., Connor K.M., Zhang Y., et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurol. 2014;83:958–66. Doi: 10.1212/WNL.0000000000000771.
24. Marcus R., Goadsby P.J, Dodick D.W., et al. BMS-927711 for the acute treatment of migraine: A double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34:114–25. Doi: 10.1177/0333102413500727.
25. Voss T., Lipton R.B., Dodick D.W., et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887–98. Doi: 10.1177/0333102416653233.
26. Lipton R.B., Croop R., Stock E.G., et al. Rimegepant, an Oral Calcitonin Gene–Related Peptide Receptor Antagonist, for Migraine. N Engl J Med. 2019;381(2):142–49. Doi: 10.1056/nejmoa1811090.
27. URL: https://clinicaltrials.gov/ct2/show/NCT02848326
28. Bigal M.E., Walter S., Rapoport A.M. Therapeutic antibodies against CGRP or its receptor. Br J Clin Pharmacol. 2015;79(6):886–95. Doi: 10.1111/bcp.12591.
29. Silberstein S., Lenz R., Xu C. Therapeutic Monoclonal Antibodies: What Headache Specialists Need to Know. Headache. J Head Face Pain. 2015;55(8):1171–82. Doi: 10.1111/head.12642.
30. Tepper S.J. History and Review of anti-Calcitonin Gene-Related Peptide (CGRP) Therapies: From Translational Research to Treatment. Headache. J Head Face Pain. 2018;58( Suppl. 3):238–75. Doi: 10.1111/head.13379.
31. Sun H., Dodick D.W., Silberstein S., et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebocontrolled, phase 2 trial. Lancet. Neurol. 2016;15:382–90. Doi: 10.1016/S1474-4422(16)00019-3.
32. Dodick D.W., Ashina M., Brandes J.L., et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026–37. Doi: 10.1177/0333102418759786.
33. Goadsby P.J., Reuter U., Hallström Y., et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123–22. Doi: 10.1056/NEJMoa1705848.
34. Tepper S.J., Ashina M., Reuter U., et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. Neurol. 2017;16:425–34. Doi: 10.1016/S1474-4422(17)30083-2.
35. Bigal M.E., Dodick D.W., Rapoport A.M., et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet. Neurol. 2015;14:1081–90. Doi: 10.1016/S1474-4422(15)00249-5.
36. Dodick D.W., Silberstein S.D., Bigal M.E., et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999–2008. Doi: 10.1001/jama.2018.4853.
37. Bigal M.E., Edvinsson L., Rapoport A.M., et al. Silberstein SD (2015a) safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet. Neurol. 2015;14:1091–100.
38. Silberstein S.D., Dodick D.W., Bigal M.E., et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113–22. Doi: https://doi.org/10.1056/nejmoa1709038.
39. Dodick D.W., Goadsby P.J., Spierings E.L., et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin generelated peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–92. Doi: 10.1016/S1474-4422(14)70128-0.
40. Skljarevski V., Oakes T.M., Zhang Q., et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA. Neurol. 2018;75:187–93. Doi: 10.1001/jamaneurol.2017.3859.
41. Stauffer V.L., Dodick D.W., Zhang Q., et al. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA. Neurol. 2018;75:1080–88. Doi: 10.1001/jamaneurol.2018.1212.
42. Detke H.C., Goadsby P.J., Wang S., et al. Galcanezumab in chronic migraine: the randomized, double-blind, placebocontrolled REGAIN study. Neurol. 2018;91:1–11. Doi: https://doi.org/10.1212/ WNL.0000000000006640
43. Dodick D.W., Goadsby P.J., Silberstein S.D., et al. ALD403 study investigators. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet. Neurol. 2014;13:1100–107.
44. A Multicenter Assessment of ALD403 in Frequent Episodic Migraine (PROMISE 1) https://clinicaltrials.gov/ct2/show/NCT02559895 Evaluation of ALD403 (Eptinezumab) in the Prevention of Chronic Migraine (PROMISE 2) https://clinicaltrials.gov/ct2/show/NCT02974153 Eptinezumab Alder Biopharmaceuticals https://www.alderbio.com
45. Do T.P., Guo S., Ashina M. Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain. 2019;20(1). Doi: 10.1186/s10194-019-0974-3.
46. Ashina M., Goadsby P.J., Reuter U., et al. Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia. 2019;033310241985408. Doi: 10.1177/0333102419854082.
47. Ning X., Cohen J., Bennett N., et al. Long-Term Safety of Fremanezumab: Results of a 1-Year Study. Neurol. 2019;92(Suppl. 15).
48. Schwedt T., Reuter U., Tepper S.J., et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain. 2018;19(1):92. Doi: 10.1186/s10194-018-0923-6.
49. Bigal M.E., Dodick D.W., Krymchantowski A.V., et al. TEV-48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology. 2016;87:41–8. Doi: 10.1212/WNL.0000000000002801.
50. Kudrow D.B. Bigal, Eptinezumab Achieved Meaningful Reductions in Migraine Activity As Early As Day 1 and Were Sustained Through Week 12: Results From PROMISE-2 (Prevention Of Migraine via Intravenous eptinezumab Safety and Efficacy-2) Phase 3 Trial in Chronic Migraine. Neurology. 2016;87.
51. Kudrow D., Lipton R., Silberstein S., et al. Eptinezumab for Prevention of Chronic Migraine: Results of 2 Infusions in the Phase 3 PROMISE-2 (Prevention of Migraine via Intravenous Eptinezumab Safety and Efficacy–2) Trial. Neurol. 2019;92(Suppl. 15).
52. Sacco S., Bendtsen L., Ashina M., et al. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1). Doi:10.1186/s10194-018-0955-y.
53. Haanes K.A., Edvinsson L. Pathophysiological Mechanisms in Migraine and the Identification of New Therapeutic Targets. CNS Drugs. 2019. Doi:10.1007/s40263-019-00630-6.
Об авторах / Для корреспонденции
Автор для связи: К.В. Скоробогатых, Университетская клиника головной боли, Москва, Россия; e-mail: post.kirill@gmail.com
Адрес: 121467, Россия, Москва, ул. Молодогвардейская, 2, корп. 1




