Введение
Фторпиримидины являются высокоэффективными и часто назначаемыми противоопухолевыми препаратами, которые используются в широком диапазоне схем химиотерапии (ХТ) для лечения различных типов злокачественных новообразований. Кардиотоксичность может быть жизнеугрожающим осложнением применения противоопухолевых лекарственных препаратов, что приводит к отказу от эффективной ХТ, тем самым сокращая продолжительность жизни пациентов. Кардиоваскулярная токсичность, вызванная фторпиримидинами, впервые описана в 1969 г., однако до сих пор недостаточно изучена. Возможные механизмы включают повреждение эндотелия, спазм и тромбоз коронарных сосудов, а также прямое токсическое воздействие на кардиомиоциты.
По оценке экспертов Европейского общества кардиологов (ESC), частота развития ишемии миокарда, вызванной фторпиримидинами, составляет до 10% в зависимости от пути введения, дозировки и схемы лечения [1, 2]. ESC подчеркнули возможность недооценки повреждения миокарда при лечении фторпиримидинами [1]. Поскольку последние наиболее часто применяются в лечении аденокарцином желудочно-кишечного тракта, мы проанализировали кардиотоксичность в данной группе пациентов.
Методы
Проанализированы данные группы пациентов с различными типами сóлидных опухолей желудочно-кишечного тракта, получавших лечение на основе фторпиримидинов, у которых была отмечена кардиотоксичность (табл. 1).
Всем пациентам лечение проводили в НМИЦ онкологии им. Н.Н. Блохина с февраля 2021 по февраль 2022 г.
Критерием проявления кардиотоксичности служило внезапное развитие симптомов, соответствующих клинической картине острого коронарного синдрома, во время или сразу после инфузии 5-ФУ/в период приема капецитабина: одышка, загрудинные боли, ишемические изменения на электрокардиограмме (ЭКГ), нарушения ритма сердца, рост кардиоферментов в динамике.
Результаты
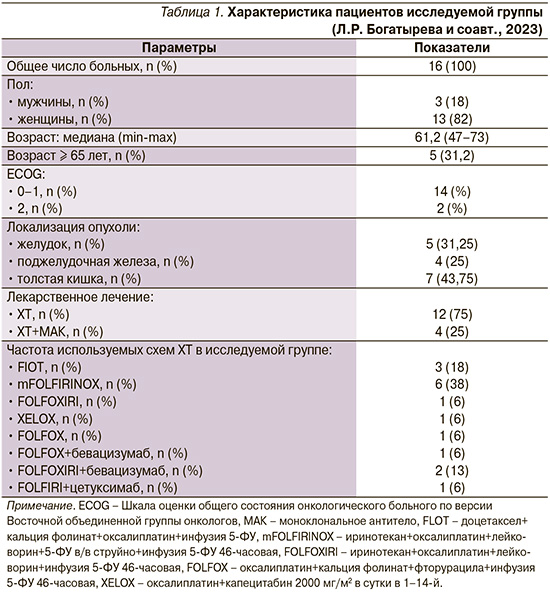
Исследуемая группа (табл. 1) состояла из 16 человек, из них 13 женщин и 3 мужчин, средний возраст составил 61,2 года (диапазон – 47–73). Рак толстой кишки диагностирован у 8 пациентов, рак желудка у 5 и рак поджелудочной железы у 4. Лечение по схеме FLOT получили 3 человека, mFOLFIRINOX – 6, FOLFOXIRI – 1, XELOX – 1 и FOLFOX – 1 человек. ХТ в комбинации с моноклональными антителами получили 4 больных: FOLFOX+бевацизумаб – 1 пациент, FOLFOXIRI+бевацизумаб – 2, FOLFIRI+цетуксимаб – 1 пациент.
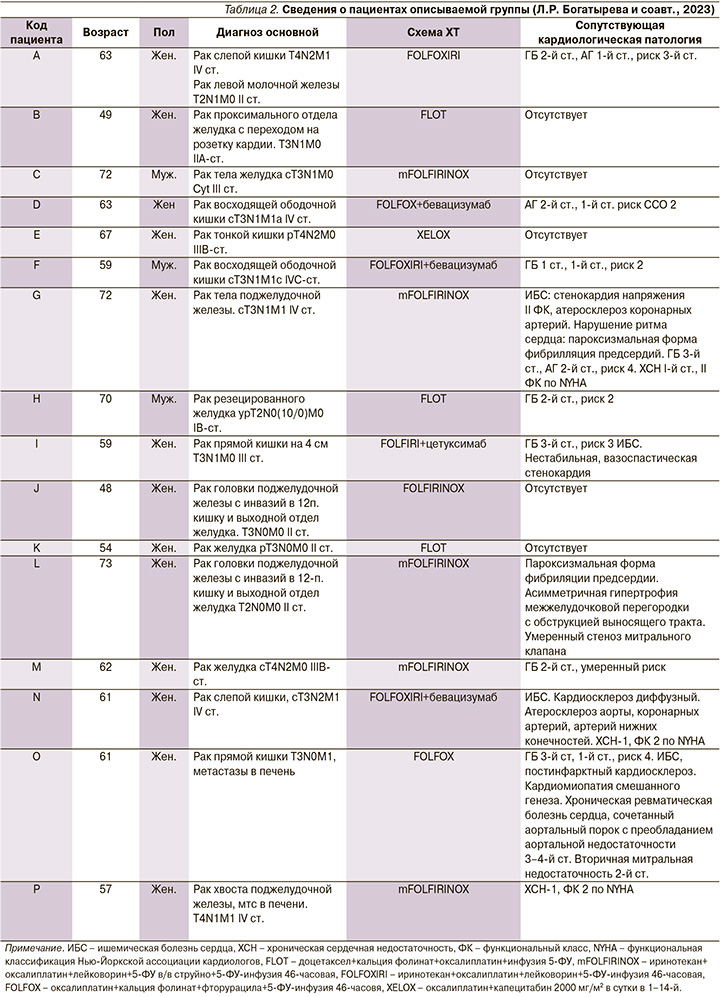
Сопутствовавшие сердечно-сосудистые заболевания были выявлены у 11 пациентов: гипертоническая болезнь (ГБ) у 9 пациентов, ишемическая болезнь сердца у 5, хронические пароксизмальные нарушения ритма сердца у 2. У одного пациента имелись хроническая ревматическая болезнь сердца, сочетанный аортальный порок. Все пациенты получали соответствующую кардиальную терапию (табл. 2).
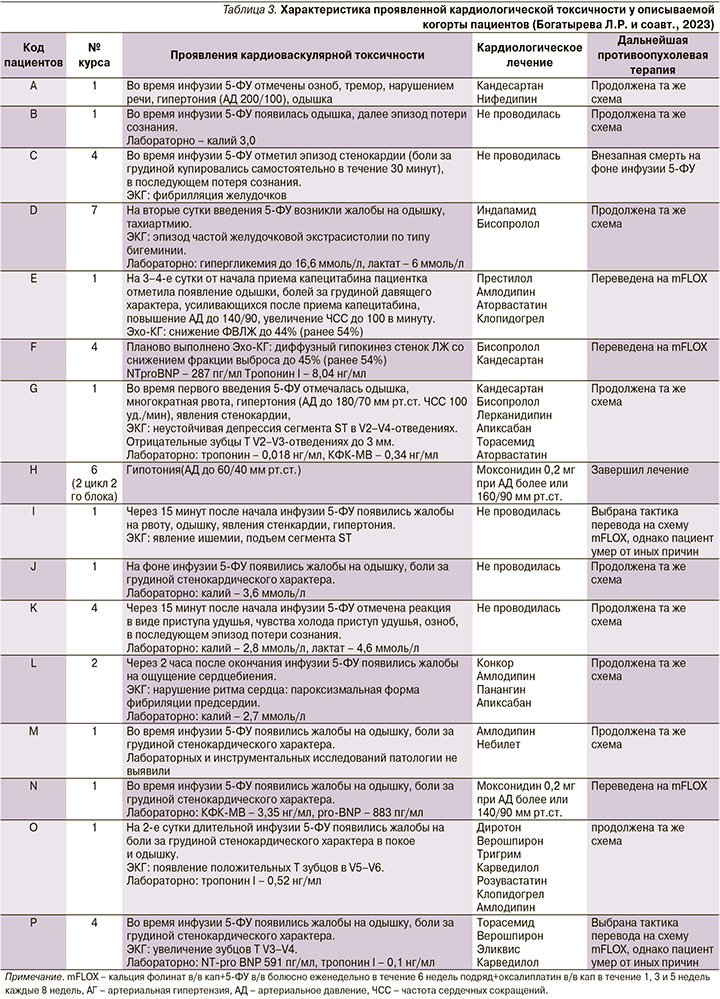
Самые частые зарегистрированные симптомы в данной выборке: одышка – 12 (75%) пациентов, загрудинные боли – 9 (56%), из них не было изменений на ЭКГ у 3 пациентов. В 4 (25%) случаях отмечены разнообразные нарушения ритма сердца: в 2 – частая желудочковая экстрасистолия (по типу бигемении), в 1 – пароксизм фибрилляции предсердий и в 1 – стойкое фатальное нарушение ритма в виде фибрилляции желудочков. Повышенный уровень тропонина I в сыворотке крови зарегистрирован в 2 (12,5%) случаях.
Снижение фракции выброса левого желудочка наблюдалось у 2 (12,5%) пациентов, у одного из них появлялась кардиальная симптоматика в виде возникновения болей за грудиной давящего характера, увеличивающихся в интенсивности после приема капецитабина. Частым симптомом была кратковременная потеря сознания – 3 (18%). Повышение уровней тропонина и NTproBNP в крови зарегистрировано у 4 (25%) пациентов (табл. 3).
У 9 (56%) пациентов кардиотоксичность отмечена во время проведения первого курса лечения, на втором, четвертом и последующих курсах – у 6,25%, 25,00 и 13,00% больных соответственно.
В 1 (6,25%) из 16 случаев кардиотоксичности зарегистрирован летальный исход: внезапная смерть во время первой инфузии 5-ФУ. В остальных 15 случаях после возникновения симптомов кардиотоксичности инфузия 5-ФУ была прекращена. Все пациенты получили соответствующую корригирующую терапию в условиях отделения интенсивной терапии. Выполнена коррекция электролитного баланса у пациентов с гипокалиемией, АГ была купирована антигипертензивными препаратами, пациентам с клинической картиной острого коронарного синдрома проведена антикоагулянтная терапия и назначены инфузии нитратов, пациентам с зарегистрированным впоследствии снижением фракции выброса левого желудочка была прекращена противоопухолевая терапия до восстановления показателя с последующим продолжением ХТ в течение 2–3 недель после проявленного эпизода токсичности.
После купирования последствий кардиотоксичности 9 (56,25%) из 16 пациентов продолжили лечение по той же схеме, 3 (18%) переведены на режим со струйным введением 5-ФУ (FLOX); в отношении 1 (6,25%) пациента, получавшего периоперационную терапию, принято решение о завершении послеоперационного блока ХТ на 2-м курсе из-за развившейся токсичности, по поводу 2 (12,5%) пациентов принято решение о смене режима ХТ, однако данные пациенты лечение начать не успели, т.к. оба погибли от иных причин в промежутке до начала терапии: один от острой почечной недостаточности, причина смерти второго – неизвестна.
Обсуждение
По данным различных авторов, частота кардиотоксичности при лечении фторпиримидинами колеблется в широком диапазоне, составляя от 1,9% [3] до 29,5% [4] для 5-ФУ и от 2,8% [5] до 34,6% [6] для капецитабина. Это можно объяснить применением разных режимов лечения, различием в критериях диагностики кардиотоксичности, в скрининговом обследовании, различной популяцией больных и различной их настороженностью в отношении симптомов кардиотоксичности [2]. Особое внимание при лечении пациентов следует уделять больным первые 72 часа после начала внутривенной инфузии фторурацила, а при применении капецитабина – первые 96 часов приема [7].
К факторам риска возникновения фторпиримидин-ассоциированной кардиотоксичности мы можем отнести конкурирующую сердечно-сосудистую патологию, вызывающую примерно 4-кратное повышение риска кардиоваскулярного события [8]. В проспективном исследовании показана связь возникновения кардиологических осложнений у женщин, употребляющих алкоголь [9].
Бевацизумаб обладает таким видом кардиоваскулярной токсичности, как стойкая бессимптомная АГ, которая может повышать риск развития кардиологических осложнений на фоне приема фторпиримидинов [10]. Зарегистрированная частота АГ высокой степени колеблется от 1,8 до 22% [11]. Имеются данные о развитии бевацизумаб-индуцированной АГ I–III степеней по шкале СТСАЕ и I–II степеней АГ – по шкале Европейского общества кардиологов (ESC) на фоне применения комбинированных режимов лекарственной терапии (полихимиотерапия+бевацизумаб) [12]. Больным с высоким риском развития гипертензии, представленным в данном исследовании, противоопухолевое лечение проводили на фоне стандартной комплексной антигипертензивной терапии, которая, по нашему мнению, и позволила избежать явлений кардиотоксичности. С этим фактом, по всей вероятности, связано отсутствие пациентов, получавших бевацизумаб, у которых единственным признаком кардиологической токсичности являлась гипертензия.
Клинические проявления фторпиримидиновой кардиотоксичности чрезвычайно разнообразны. По данным литературы, самыми частыми клиническими проявлениями являются стенокардия (45%) и аритмии (33%), инфаркт миокарда – 22% [13]. При инструментальных методах диагностики показано значимое увеличение бессимптомной ишемии миокарда [14]. Достоверно установлено, что терапия фторпиримидинами увеличивает длительность интервала QT, которое сохраняется до 6 месяцев после окончания терапии [15–16].
В литературных источниках представлены случаи развития миокардита после первого курса лечения 5-ФУ с набором типичных симптомов [17]. Некоторые авторы описывают такое редкое явление, как кардиомиопатия Такоцубо, развившуюся после ХТ 5-ФУ [18, 19]. Токсичность фторпиримидинов может сочетаться с другими видами токсичности. Так, представлен случай одновременной кардиотоксичности и инсультоподобной нейротоксичности у пациента, получавшего лечение по схеме FOLFOX. Авторы предполагают, что спазм коронарных артерий и сосудов головного мозга, вызванный 5-ФУ, может вызывать одновременные кардиальные и неврологические нарушения [20].
Роль proBNP и тропонина I оценена в недавнем исследовании [9]. Хотя тропонин I и proBNP обычно используются в клинической практике для диагностики и мониторинга эффективности лечении заболеваний сердца, результаты исследования показали, что они не могут быть эффективно использованы для прогнозирования кардиологической токсичности фторпиримидинов. В частности, ни один из них существенно не изменил свою экспрессию у пациентов с зарегистрированными случаями кардиологической токсичности ни в одном из циклов лечения [9].
Патогенез кардиологической токсичности фторпимидинов полностью не изучен. Исследования на животных показали, что 5-ФУ индуцировал патологические изменения в миокарде, а также в эндотелии стенок артериальных сосудов, получены доказательства системного повреждения эндотелия [21, 22]. Повреждения в миокарде, по-видимому, зависели от введенной дозы 5-ФУ, поскольку высокие дозы приводили к более выраженным повреждениям [22]. Сопутствующее лечение пробуколом, гиполипидемическим препаратом с сильными антиоксидантными свойствами, нивелировало действие 5-ФУ на эндотелий [21], в то время как сопутствующее лечение далтепарином, низкомолекулярным гепарином, приводило к несколько иной картине повреждения эндотелия на 3-й день, уменьшаясь на 7-й, но снова усиливаясь к 14-му дню [23]. Далтепарин предотвращал образование фибрина и в меньшей степени – агрегацию тромбоцитов [23]. Остается неясным, в какой степени гистопатологические особенности, продемонстрированные в исследованиях на животных, могут быть обнаружены у пациентов, испытывающих клинические признаки кардиотоксичности, поскольку пациентам с симптомами кардиотоксичности не проводились биопсийные исследования.
Агрегация тромбоцитов и образование фибрина в местах повреждения эндотелия при сканирующей электронной микроскопии эндотелия сосудов кроликов позволили предположить, что патофизиологический механизм кардиотоксичности, индуцированной 5-ФУ, включает тромбогенный эффект, вторичный по отношению к повреждению эндотелия [21–25]. Однако отсутствие окклюзий сосудов у многих пациентов, проходящих коронарную ангиографию по поводу боли в груди, вызванной 5-ФУ, не подтверждает тромбоз как основной механизм [26–33]. С другой стороны, состояние продолжающегося внутрисосудистого свертывания известно из исследований свертывающе-фибринолитической системы. Прокоагулянтное состояние часто встречается у онкологических больных и вызывается прокоагулянтными факторами, продуцируемыми опухолью, и цитокинами, происходящими из опухолевых клеток [32].
Была предложена теория спазма сосудов, ведущего к ишемии миокарда, поскольку коронарная ангиография не выявила стенозов значительной степени у пациентов с острой кардиотоксичностью, вызванной 5-ФУ [26–31, 34, 35]. В то же время при коронарной ангиографии визуализирован вазоспазм коронарных артерий [33, 36, 37]. Имеются данные исследований, в которых после инъекции 5-ФУ зарегистрирована периферическая вазоконстрикция плечевой артерии [38, 39]. Вызванное 5-ФУ сужение сосудов было кратковременным, повторялось при повторных инъекциях 5-ФУ и устранялось применением нитроглицерина. Исследователи предположили, что сужение сосудов, измеряемое периферически после инъекции 5-ФУ, происходит также в коронарных артериях [39]. Моссери и другие изучали 5-ФУ-индуцированную вазоконстрикцию in vitro с использованием изолированных колец аорты, вырезанных у кроликов. Распространенность сужения сосудов коррелировала с молярной концентрацией 5-ФУ, а степень была пропорциональна концентрации 5-ФУ. Степень вазоконстрикции была аналогичной для колец аорты с функционально сохраненным эндотелием и колец аорты с предварительно разрушенным эндотелием, что указывает на то, что поврежденный эндотелий не был необходимым условием для развития 5-ФУ-индуцированной вазоконстрикции. Данный эффект устранялся нитроглицерином [40].
Для подтверждения вазоспазма в коронарных артериях необходимы инвазивные методы, такие как катетеризация сердца и коронарная ангиография во время инфузии. В то время как сужение сосудов наблюдается сразу после инъекции 5-ФУ, клинические проявления кардиотоксичности часто возникают в конце инфузии или через несколько часов или дней [41]. Более того, кардиотоксичность может возникать после нескольких инфузий 5-ФУ или длительного приема капецитабина. Следовательно, остается выяснить, при каких обстоятельствах вазоконстрикция, вызванная 5-ФУ, приводит к клиническим признакам кардиотоксичности.
Предложенные пути патогенеза кардиологической токсичности фторпимидинов не взаимоисключающие, и каждый из них может вносить вклад в сердечно-сосудистую дисфункцию, приводящую к клинической картине кардиотоксичности. Для выяснения патогенеза этого побочного эффекта необходимы дальнейшие исследования, такие как исследования на линиях сердечных и эндотелиальных клеток животных, исследование на людях с непрерывным мониторированием ЭКГ одновременно с измерениями диаметров плечевой артерии, исследования перфузии миокарда с помощью магнитно-резонансного сканирования или ПЭТ-рубидиевого сканирования сердца у пациентов с признаками кардиотоксичности.
Лечение кардиологической токсичности, вызванной фторпиримидинами, проводится в соответствии с клинической картиной. Поскольку основным механизмом развития кардиальных симптомов остается спазм коронарных сосудов, многие специалисты предпочитают использовать препараты, действующие преимущественно на коронарные артерии, а именно блокаторы кальциевых каналов и нитраты [42–44]. К примеру, описана серия наблюдений больных фторпиримидин-индуцированными кардиологическими осложнениями, продолживших успешное лечение фторпиримидинами на фоне профилактического приема дилтиазема [45].
Вопрос возможности и безопасности последующего использования фторпиримидинов в каждом конкретном случае требует индивидуального решения и активной кардиологической профилактики. В литературе опубликованы данные, анализирующие смертность при повторном введении 5-ФУ после зафиксированной кардиотоксичности. В ранних работах смертность составила 13% [12]. Однако в недавно опубликованных результатах проспективного исследования [9] повторное лечение фторпиримидинами в тех же дозах и режимах, что и до случая регистрации кардиотоксичности, предпринято в отношении 90% пациентов. Только 22% из них получили сопутствующую кардиотерапию ингибиторами ангиотензинпревращающего фермента, антиаритмическими препаратами или β-адреноблокаторами. Этим пациентам не потребовалось ни дальнейших вмешательств, ни снижения дозы.
Однако повторное лечение на основе 5-ФУ/капецитабина без кардиопротекторной профилактики пациентам, у которых развилась кардиоваскулярная токсичность, связанная с фторпиримидинами, не рекомендуется, поскольку имеются данные, согласно которым симптомы кардиоваскулярной токсичности рецидивируют у 82–100% этих пациентов с 18%-ной смертностью [47]. Авторы данного исследования показали, что у пациентов, продолживших лечение на основе 5-ФУ/капецитабина с интенсивной кардиопрофилактикой, был снижен риск смерти (отношение рисков – 0,42) и зарегистрирована тенденция к снижению прогрессирования основного заболевания (отношение рисков – 0,60) по сравнению с пациентами, которые прекратили лечение на основе фторпиримидинов. Число случаев повторных болей за грудиной у этих пациентов также значительно снизилось: с 67 до 19% [46].
Ряд литературных данных свидетельствует, что кардиотоксичность 5-ФУ проявляется при его введении в виде непрерывной инфузии чаще, чем при болюсном введении [2, 12], поэтому, если после зарегистрированного случая фторпиримидиновой кардиотоксичности пациенту необходимо продолжить лечение режимами, включающими 5-ФУ, стоит рассмотреть замену инфузионного введения 5-ФУ струйным с подбором соответствующих схем. В литературе представлено исследование на европейской популяции пациентов колоректальным раком, результаты которого показали, что в случае развития кардиоваскулярной токсичности на фоне капецитабина можно рассмотреть вопрос о назначении S-1 в соответствующих дозировках [47].
Вопрос эффективной профилактики кардиологической токсичности фторпиримидинов остается в настоящее время предметом изучения.
Заключение
Кардиотоксичность фторпиримидинов является нечастым, но реальным явлением, которое не зависит от дозы и может быть связано с непрерывной инфузией. Хотя патогенез кардиотоксичности, вызванной фторпиримидинами, остается неясным, основным механизмом принято считать спазм коронарных сосудов.
При назначении лечебных схем, включающих фторпиримидины, следует уделять особое внимание сопутствующей сердечно-сосудистой патологии, вследствие того что отягощенный сердечно-сосудистый анамнез повышает риск развития фторпиримидиновой кардиотоксичности. Пациенты, получающие лечение на основе фторпиримидинов, должны находиться под пристальным наблюдением. При любых настораживающих врача симптомах, в особенности кардиальных, введение 5-ФУ должно быть прекращено. Дальнейшие действия должны быть направлены на быстрое выявление симптомов острого коронарного синдрома: наличие характерных жалоб, одышки, перебоев в работе сердца, изменений АД. Должна быть записана свежая ЭКГ. Последующая тактика ведения пациента, в т.ч. и решение вопроса о необходимости проведения коронарографии, предполагает взаимодействие онколога с врачом интенсивной терапии и кардиологом.
Повторное введение фторпиримидинов не рекомендуется, за исключением случаев, когда они значительно улучшают прогноз, но только после тщательного обследования сердечно-сосудистой системы и проведения профилактики. При продолжении лечения с применением 5-ФУ стоит рассмотреть перевод на болюсное его введение, замену капецитабина на S1. Данным пациентам, особенно с высоким риском развития кардиотоксичности, рекомендуются усиленная кардиологическая терапия и интенсивный мониторинг.



