Введение
Саркомы мягких тканей (СМТ) – гетерогенная группа мезенхимальных опухолей, наиболее часто встречающаяся среди детей. В 2017 г. в России зарегистрировано 3567 случаев заболевания данной патологией, что составляет менее 1% всех злокачественных новообразований у взрослого населения [1, 2].
СМТ характеризуются малой чувствительностью к лекарственной терапии и крайне неблагоприятным прогнозом. Летальность в течение первого года с момента установления диагноза составляет 19,7% [1]. Несмотря на значительные успехи в лечении большинства злокачественных новообразований, лечение диссеминированной СМТ по-прежнему остается трудной задачей. При IV стадии заболевания медиана общей выживаемости (ОВ) на фоне стандартных методов лечения составляет не более 12–18 месяцев и зависит главным образом от гистологического подтипа СМТ [3, 4].
Одним из многообещающих препаратов стал пазопаниб – пероральный низкомолекулярный мультикиназный ингибитор рецепторов эндотелиального фактора роста-1, -2, -3 (VEGFR-1, -2 и -3), рецептора фактора роста тромбоцитов-α и -β (PDGFR-α и -β), рецептора фактора роста фибробластов-1 и -3 (FGFR-1, -3), рецептора цитокина (c-Kit) [5]. Основываясь на результатах III фазы рандомизированного двойного слепого исследования PALETTE, в апреле 2012 г. FDA (Управление по санитарному надзору за качеством пищевых продуктов и медикаментов) одобрило пазопаниб в качестве 2-й и последующих линий терапии диссеминированных СМТ, за исключением липосарком и ГИСО (гастроинтестинальных стромальных опухолей) [6, 7]. Таким образом, пазопаниб стал первым и единственным ингибитором тирозинкиназы, одобренным для лечения множества гистологических подтипов СМТ.
Цель данного исследования: сравнение результатов III фазы исследования PALETTE и данных, полученных в ходе собственного клинического опыта.
Методы
Для анализа были включены данные о пациентах, получавших таргетную терапию пазопанибом по поводу различных гистотипов СМТ (кроме экстраскелетной миксоидной хондросаркомы) в качестве 2-й и последующих линий терапии с 2013 по 2021 г. в ФГБУ «НМИЦ онкологии им. Н.Н. Петрова». Проведен анализ информации о возрасте, поле пациента, морфологическом подтипе СМТ, режиме и количестве линий предыдущей лекарственной терапии. Конечной точкой нашего исследования было определение выживаемости без прогрессирования (ВБП). В дополнение оценивались длительность терапии пазопанибом, а также частота объективного ответа. Статистическая обработка и анализ данных был проведен в программе IBM SPSS Statistics (версия 28.0.0.0).
Результаты
Мы проанализировали амбулаторные карты 47 пациентов, которые получали или продолжают получать таргетную терапию пазопанибом в ФГБУ «НМИЦ онкологии им. Н.Н. Петрова» с 2013 г. по настоящее время (табл. 1). Поскольку целью исследования было непрямое сравнение результатов регистрационного исследования PALETTE с результатами собственного клинического опыта, из анализа были исключены 4 пациента, получивших пазопаниб в качестве 1-й линии лекарственного лечения, и 5 пациентов с экстраскелетной миксоидной хондросаркомой, которые также не были включены в исследование PALETTE. Таким образом, проанализированы данные 38 больных. Количество женщин значительно преобладало (73,7 и 26,3% соответственно). Средний возраст пациентов составил 47,5 года (диапазон от 20 до 72 лет).
Всего было выявлено 9 различных гистологических подтипов СМТ. Наиболее часто встречаемым вариантом была лейомиосаркома (39,5% больных). Среди других морфологических подтипов СМТ – это недифференцированная саркома (18,4%), синовиальная саркома (13,2%), фибросаркома (7,9%) и альвеолярная саркома (7,9%), MPNST (5,3%); еще у 3 (7,9%) больных был верифицирован иной гистотип СМТ.
Основным поводом к назначению пазопаниба стало прогрессирование на фоне химиотерапии антрациклинами (86,8%) или таксанами (47,4%) либо на фоне терапии аутологичной дендритно-клеточной вакциной (АДКВ) (7,9%). Другие схемы лечения, в т.ч. метрономную терапию циклофосфамидом в сочетании с метотрексатом, ранее получали 11 (28,9%) больных. Таким образом, количество предыдущих линий лекарственной терапии составило от 1 до 4; однако чаще всего пазопаниб назначался в качестве 2-й линии (50,0%).
Медиана длительности лечения пазопанибом составила 8,4 месяца (диапазон от 0,9 до 68,9 месяца). Так, у пациента с альвеолярной саркомой, ранее получавшего режим MAID, прогрессирование заболевания зарегистрировано спустя 68,9 месяца приема пазопаниба.
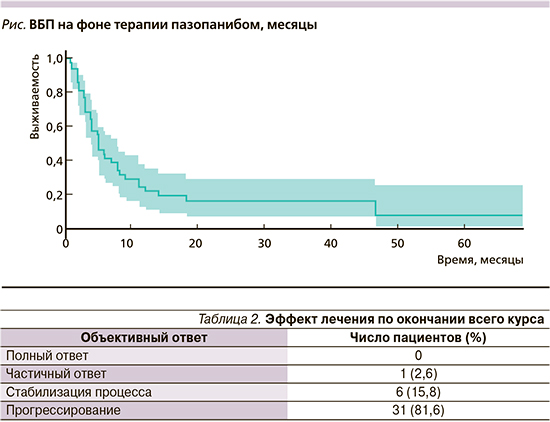
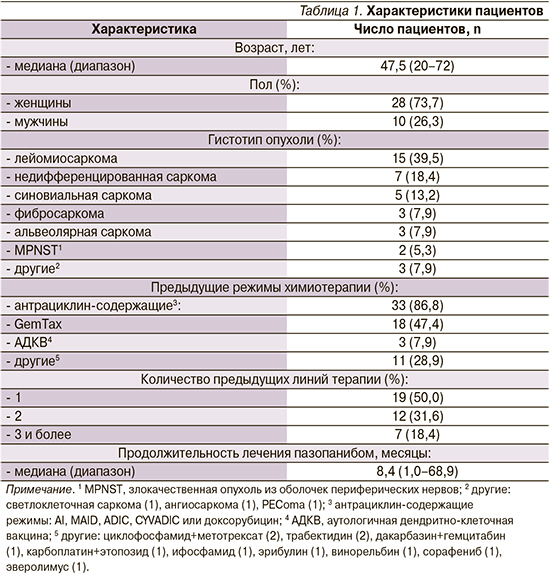
Частота объективного ответа составила 18,4%: у 1 (2,6%) пациента зарегистрирован частичный ответ, у 6 (15,8%) – стабилизация опухолевого процесса (табл. 2). Медиана ВБП составила 4,0 месяца (95% доверительный интервал: 3,0–8,0; рис. 1). Примечательно, что пазопаниб продемонстрировал эффективность даже при 4-й и последующих линиях терапии. Лечение пазопанибом в целом переносилось удовлетворительно, тяжелой степени токсичности не выявлено.
Обсуждение
Первоначально пазопаниб разрабатывался как низкомолекулярный ингибитор рецепторов фактора роста эндотелия сосудов, однако на доклинической фазе исследования выявлено, что пазопаниб оказывает противоопухолевый эффект посредством ингибирования как ангиогенных, так и онкогенных сигнальных путей. Первые исследования пазопаниба были направлены на выявление его активности в отношении пациентов с почечно-клеточной карциномой. А после его одобрения для лечения распространенного почечно-клеточного рака пазопаниб был исследован в качестве лечения СМТ [7].
На доклинической фазе исследований пазопаниб продемонстрировал эффективность в отношении ингибирования рецепторов сосудистого эндотелиального фактора роста (VEGFR1 и VEGFR3), а также других близкородственных тирозинкиназ, включая рецепторы тромбоцитарного фактора роста (PDGFRB), KIT, рецепторы фактора роста фибробластов (FGFR1) и колониестимулирующий рецептор фактора 1 (CSF1R). Фармакодинамические исследования биологической активности этого лекарственного средства показали, что противоопухолевый эффект пазопаниба может быть опосредован не только ингибированием ангиогенеза, но и прямым воздействием на опухолевые клетки [8].
На основании результатов доклинической фазы стартовало исследование I фазы, в котором приняли участие 63 больных СМТ и другими солидными опухолями в возрасте от 3,8 до 23,9 года [9]. Данные фармакокинетики показали, что устойчивое воздействие достигалось в дозах 800 мг или более при приеме 1 раз в день per os. По данным оценки предварительной клинической активности у 3 пациентов зафиксирован частичный ответ по критериям RECIST (у 2 с почечноклеточный раком и у 1 с аденокарциномой поджелудочной железы), в то время как у 14 больных наблюдалась стабилизация заболевания продолжительностью не менее 6 месяцев. Следует отметить, что среди них были 2 пациента с хондросаркомой, 1 с лейомиосаркомой и 1 с ГИСО. Наиболее частым нежелательным явлением оказалась артериальная гипертензия (3-я степень наблюдалась у 25% пациентов), реже – диарея, депигментация волос, тошнота, анорексия и астения. По результатам исследования, у 4 из 9 больных саркомой, получавших пазопаниб, наблюдалась длительная стабилизация заболевания. Это послужило толчком для продолжения исследований данного препарата для оценки активности в лечении сарком.
Первым исследованием по изучению эффективности пазопаниба для больных мягкотканными саркомами было нерандомизированное исследование II фазы EORTC-STBSG, включившее 142 пациента с различными гистологическими подтипами сарком: лейомиосаркома, липосаркома, синовиальная саркома и гетерогенная группа «другие» [10]. Первичной конечной точкой эффективности был показатель ВБП через 12 недель, который в группе липосарком составил 26% (5/19 пациентов). В группах лейомиосарком, синовиальных сарком и в группе «другие» ВБП составила 44% (18/41 пациентов), 49 (18/37) и 39% (16/41) соответственно. Ни у одного больного не было отмечено полного регресса. Частичный ответ наблюдался у 9/136 (6,6%) пациентов: 5 – в группе синовиальных сарком, 3 – в группе «другие» и 1 – в группе лейомиосарком. Медиана ВБП составила 80, 91, 161 и 91 день, медиана ОВ – 197, 354, 310 и 299 дней в группе липосарком, лейомиосарком, синовиальных сарком и «другие» соответственно.
Основываясь на результатах исследования II фазы EORTC-STBSG, стартовало рандомизированное двойное слепое исследование III фазы PALETTE [11]. В исследование были включены 369 больных метастатической СМТ, из которых 246 получали пазопаниб, 123 – плацебо. Из исследования были исключены пациенты с липосаркомой, эмбриональной рабдомиосаркомой, ГИСО, выбухающей дерматофибросаркомой, саркомой Юинга, хондросаркомой и остеосаркомой. Медиана наблюдения составила 25 месяцев. Частота объективного ответа оказалась в 2 раза выше в группе пациентов, получавших пазопаниб, чем в группе плацебо, и составила 73% (6% – частичный ответ, 67% – стабилизация) и 38% (0% – частичный ответ и 38% – стабилизация) соответственно. Кроме того, больные, получавшие пазопаниб, продемонстрировали значительно лучшую медиану ВБП: 4,6 месяца по сравнению с 1,6 для группы плацебо (95% ДИ: 0,24–0,40; р<0,001). Однако не было выявлено статистически достоверных различий ОВ в обеих группах: 12,5 месяцев для пазопаниба и 10,7 – для плацебо (95% ДИ: 0,67–1,11; р=0,25).
В нашем исследовании медиана ВБП составила 4,0 месяца, что сопоставимо с результатом регистрационного исследования PALETTE. Спектр побочных эффектов также соответствовал ранее опубликованным данным.
Заключение
Полученные в ходе в реальной клинической практики данные сопоставимы с результатами ключевого рандомизированного клинического исследования PALETTE. Это подтверждает эффективность назначения пазопаниба для больных диссеминированной СМТ.



