Введение
В России, согласно данным Федеральной службы по надзору в сфере защиты прав потребителей и благополучия человека, в доковидный период число заболевших острой респираторной вирусной инфекцией (ОРВИ), в т.ч. и гриппом, детей ежегодно регистрировали примерно на уровне 80–120 тыс. заболеваний на 100 тыс. населения детского возраста (в 3,3–6 раз выше, чем у взрослых) без тенденции к снижению [1, 2]. При этом частота встречаемости зарегистрированных случаев гриппа у детей (в пересчете на 100 тыс.) была в 1,9–2,8 раза более высокой, чем у всего населения (табл. 1).
По данным Национального центра по гриппу (НЦГ) Всемирной организации здравоохранения (ВОЗ), спектр возбудителей ОРВИ был разным, обусловленным сезоном и возрастом наблюдаемых пациентов: среди дошкольников это адено-, рино-, респираторно-синцитиальный (РС) и парагриппозный вирусы, у школьников – адено- и метапневмовирусы [3, 4]. Примерно в 25–30% случаев, особенно во вновь созданных коллективах (группа ДДУ, первый класс школ), имело место одновременное участие нескольких возбудителей, обусловленное эффектом «перемешивания». Вирус гриппа доминировал у пациентов любого возраста только во время эпидемического подъема гриппа (см. рисунок).
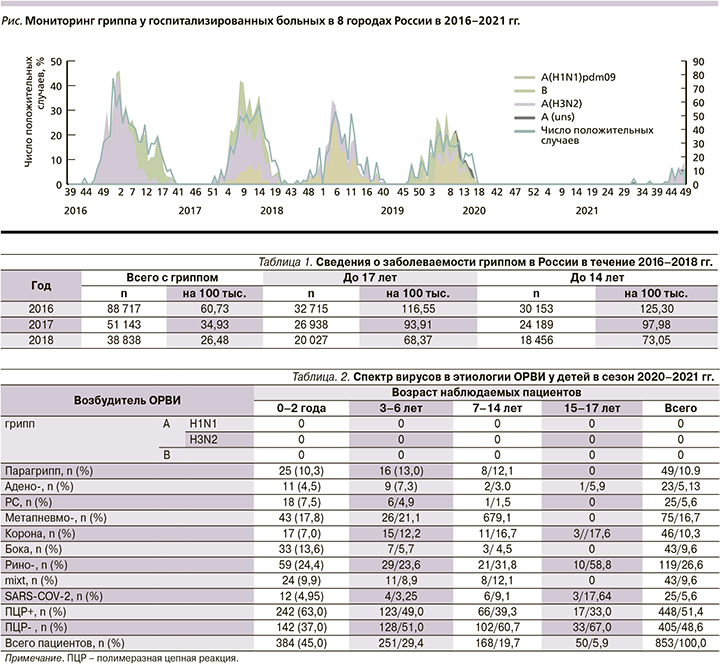
Появление в 2019 г. нового возбудителя, SARS-CoV-2, ставшего причиной COVID-19, привело к вытеснению из эпидемической циркуляции других возбудителей ОРВИ, прежде всего вирусов гриппа, и отсутствию эпидемического подъема гриппа зимой 2020–2021 гг. Аналогичная эпидемическая ситуация зарегистрирована ВОЗ и в других странах мира [5, 6].
Наблюдения за этиологией и течением ОРВИ у 853 детей в возрасте от 0 до 17 лет, госпитализированных в два детских стационара (базовых для ФГБУ НИИ гриппа МЗ РФ им. А.А. Смородинцева), позволили сделать вывод, согласно которому в течение эпидемического периода с 41-й недели 2020 по 22-ю 2021 г. регистрируемые острые респираторные заболевания у обследованного контингента пациентов были только негриппозной этиологии (табл. 2).
Среди пациентов доминировали дети первых 2 лет жизни: 384 (45,0%), у которых преобладала регистрация рино-: 59 (24,4%) из 242 ПЦР+, и метапневмо-: 43 (17,8%) вирусных инфекций. Те же инфекции чаще других регистрировались в возрастной группе детей 3–6 лет. У школьников преобладали случаи заболеваний с невыясненной этиологией: 102 (60,7%) из 168 пациентов в возрасте 7–14 лет и 33 (67,0%) из 50 госпитализированных подростков 15–17 лет. Из доказанных возбудителей у них также преобладал риновирус – 21 (31,8%) из 66 ПЦР+ обследованных детей в возрасте 7–14 лет и 10 (58,8%) из 17 ПЦР+ подростков. При этом следует отметить, что участие в этиологии ОРВИ у детей в возрасте 0–2 и 3–6 лет SARS-CoV-2 вируса не было значимым: 12 (4,95%) из 242 ПЦР+ обследованных в возрасте 0–2 лет, 4 (3,25%) среди детей в возрасте 3–6 лет. Его роль повышалась при развитии заболеваний у пациентов более старшего возраста: 6 (9,1%) из 66 ПЦР+ детей в возрасте 7–14 лет и 3 (17,64%) из 17 ПЦР+ подростков.
Вместе с тем в 2020–2021 гг., по данным ВОЗ, вирусы гриппа как B, так и А не исчезли из циркуляции, а их спорадическая активность была зарегистрирована во многих странах мира [5, 6].
С августа–сентября 2021 г. вирусы гриппа начали также выявляться у заболевших и в системе Сигнального надзора (СН) за гриппом у больных в России [7]. А уже в ноябре 2021 г., по данным НЦГ ВОЗ в России при НИИ гриппа им. А.А. Смородинцева Минздрава России, во многих городах страны был зарегистрирован рост частоты диагностирования гриппа A(H3N2), По данным Еженедельного национального бюллетня по гриппу и ОРВИ, за 49-ю неделю 2021 г. уровень заболеваемости населения ОРВИ и гриппом в целом по стране повысился по сравнению с предыдущей неделей и, составив 109,9 на 10 тыс. населения, стал выше базовой линии (70,0) на 57,0% и выше еженедельного эпидемического порога на 48,5%. При исследовании материалов лабораторными методами от 6189 больных гриппом и ОРВИ зарегистрировано 927 (15,0%) случаев гриппа, в т.ч. 886 случаев гриппа A(H3N2), антигенный состав которых подобен референс-штамму A/Камбоджа/e0826360/2020 (H3N2), входящему в состав вакцин, рекомендованных ВОЗ для Северного полушария на сезон 2021–2022 гг. Кроме того, в НЦГ Москвы доказано наличие чувствительности у 26 выделенных вирусов гриппа А(H3N2) к ингибиторам нейраминидазы (осельтамивир, занамивир).
В ноябре появилось сообщение СDC CША о вспышке гриппа A(H3N2) в университетском городке в Мичигане [8]. Эти факты говорят о том, что уже в ближайшие месяцы в стране может развиться очередная эпидемия гриппа.
Кроме того, Всемирная организация здравоохранения животных (МЭБ) сообщила об учащении развития вспышек птичьего гриппа среди диких и домашних птиц, которые с мая этого года были зарегистрированы более чем в 40 странах. В октябре того же года было зарегистрировано почти 16 тыс. случаев птичьего гриппа, что предполагает повышенный риск циркуляции вируса [9].
Способность вирусов гриппа к антигенной изменчивости в виде дрейфа (точечных мутаций в гене в пределах подтипа, вызывающих ежегодные подъемы заболеваемости) или шифта (только у вирусов гриппа типа А – изменение структуры HA, и/или NA) определяет высокую восприимчивость населения и основные эпидемиологические особенности этой инфекции: повсеместное распространение, короткие интервалы между эпидемиями (1–2 года для гриппа А и 2–4 для гриппа В), вовлечение в эпидемический процесс всех возрастных групп населения. Отсутствие специфического иммунитета к шифтовым вариантам вируса гриппа типа A приводит к быстрому распространению инфекции по всему миру с увеличением числа тяжелых форм заболевания и летальных исходов, что наблюдалось во время пандемии гриппа в 2009 г. и во время эпидемического подъема гриппа в сезон 2015–2017 гг. [10, 11].
Патогенез гриппа слагается из комплекса процессов, развивающихся одномоментно или последовательно друг за другом на всех этапах репродукции возбудителей, и последующего их распространения по организму, основные из которых – это:
1. Адсорбция и внедрение вирусов гриппа в эпителиальные клетки респираторного тракта (входные ворота инфекции) при условии наличия в них специфических клеточных рецепторов, представленных для вируса гриппа сиаловыми кислотами гликопротеидов [12]. Репродукция новых вирионов сопровождается цитопатическим (по отношению к клеткам) эффектом, их разрушением и отторжением вместе с вновь образующимися вирусами.
2. Проникновение патогенов, продуктов окислительно-метаболических процессов и разрушенных эпителиальных клеток в русло крови (вирусемия, токсинемия) с развитием вазопатии (системных токсических или токсико-аллергических реакций) [13, 14].
3. Доказана возможность развития эндотелиоза (альтеративной воспалительной реакции) в виде клеточного некроза и апоптоза, повышения проницаемости капилляров, а в наиболее критических случаях – и циркуляторных нарушений при репродукции вирусов гриппа в клетках эндотелия кровеносных сосудов [15].
Возможно развитие геморрагического капилляротоксикоза, микротромбоэмболий, которые в виде застойного полнокровия головного мозга и мелких кровоизлияний в эпикард, плевру, легкие и другие органы с глубокими гемодинамическими расстройствами считаются постоянной находкой при патологоанатомическом исследовании умерших от гриппа в первые дни болезни [16].
Основной принцип успеха терапии гриппа – раннее начало сразу же при появлении первых симптомов заболевания с учетом выраженности, тяжести течения и локализации процесса, наличия осложнений, а также возраста и преморбидного фона пациента.
Следует, к сожалению, отметить, что выбор этиотропных препаратов для лечения гриппа у детей раннего возраста весьма мал. Это препараты рекомбинантного интерферона α2b (ИФН-α2b, разрешен с рождения), а также рекомендуемые детям в возрасте ≥1 года осельтамивир (суспензия) и полимерный препарат Орвирем (римантадин+альгинат натрия) в сиропе [17].
С появлением нового антигенного варианта вируса гриппа – возбудителя пандемии 2009 г., резистентного к общепринятому противовирусному препарату ремантадину, спасением оказались препараты селективного действия – ингибиторы нейраминидазы, блокирующие ключевой фермент репликации вирусов гриппа А, и В-нейраминидаза, контролирующая процессы инфицирования клеток хозяина и выхода из них вирионов. Один из них – осельтамивира фосфат (Ф. Хоффманн-Ля Рош, Швейцария), является «пролекарством», который в организме превращается в осельтамивира карбоксилат – ингибитор нейраминидазы вирусов гриппа типов A и B. FDA (Food and Drug Administration) в 2012 г. одобрило применение препарата для лечения гриппа у младенцев в возрасте от 2 недель и старше [18].
С целью лечения гриппа препарат получили более 90 млн людей, включая примерно 30 млн детей более чем 80 стран. На основании предписания ВОЗ препараты ингибиторов нейраминидазы входят в стратегический резервный фонд большинства стран Европейского Союза для профилактики и лечения гриппа при пандемии [19].
Цель настоящего исследования: обобщение результатов наблюдения над лечебной эффективностью осельтамивира (моно или в сочетании с рекомбинантным ИФН-α2b, вводимым интраназально), при гриппе у детей ≥1 года жизни, выполненных сотрудниками отделения РВИ у детей ФГБУ НИИ гриппа им. А.А. Смородинцева.
Методы
Препараты этиотропного действия:
- ингибитор нейраминидазы осельтамивир (Швейцария) Регистрационный номер капсул П № 012090/01, порошка для приготовления суспензии для приема внутрь ЛС-000550. В России разрешен к применению с лечебной целью детям ≥1 года жизни. Пациенты получали осельтамивир в двух лекарственных формах (в капсулах или в суспензии): при массе тела менее 15 кг – 30 мг, от 15 до 23 кг – 45 мг, в капсулах не больше 75 мг 2 раза в сутки в течение 3–5 дней.
- рекомбинантный ИФН-α2b (Грип-пферон) капли и спрей назальные (ООО «Фирн-М», Москва). В 1 мл препарата содержится не менее 10 тыс. МЕ ИФН, а также антиоксидант трилон-В и биологически совместимый полимер повидон-8000 [20].
Этиологию заболеваний определяли в материале из носовых ходов и носоглотки с помощью методов ПЦР (ОТ-ПЦР в режиме реального времени) c использованием комплекта реагентов по протоколу CDC) и ИФЛ-диагностики с помощью стандартных препаратов флуоресцирующих антител [21]. Статистическая обработка полученных данных проводилась на ПК Pentium IV с использованием прикладных программ Microsoft Excel 7,0, Stat Soft Statistica v 6.0
Исследование лечебной эффективности осельтамивира при гриппе у 434 детей осуществлялось сотрудниками ФГБУ в течение 2009–2019 гг. в соответствии с федеральным законом «Об обращении лекарственных средств» № 61-ФЗ от 01.09.2010 (с ред. от 01.01.2017) и Правилами Надлежащей клинической практики государств – членов ЕАЭС (Евразийский Экономический Союз).
Результаты
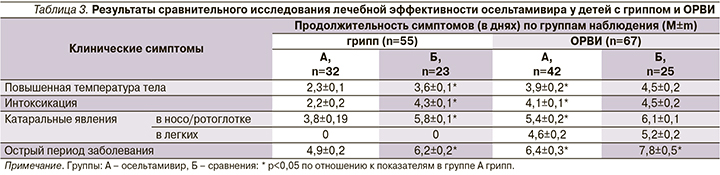
Прежде всего сравнили эффективность препарата при гриппе (55 человек ≥5 лет) и ОРВИ негриппозной этиологии (67 детей). Всего осельтамивир получили 74 человека, 48 детей вошли в состав групп сравнения, получив базовую патогенетическую терапию, назначаемую и пациентам основных групп.
Доказано, что применение ингибитора нейраминидазы осельтамивира оказалось эффективным при лечении детей с гриппом, тогда как при ОРВИ негриппозной этиологии его эффективность оказалась незначительной (табл. 3).
Проведенная оценка безвредности применения осельтамивира для больных при помощи динамики показателей клинического анализа крови, общего анализа мочи и рутинных биохимических показателей (содержание мочевины, аланинаминотрансферазы, аспартатаминотрансферазы и щелочной фосфатазы в сыворотке крови) не выявила нежелательного воздействия препарата на обследованных пациентов, что было доказано и ранее [22].
В наших исследованиях доказано, что тяжелые и осложненные формы гриппа развивались в основном у лиц с низким содержанием ИФН, особенно во входных воротах инфекции [23], что стало показанием к проведению исследования эффективности сочетанного применения осельтамивира и препарата рекомбинантного ИФН-α2b, вводимого интраназально (табл. 4).
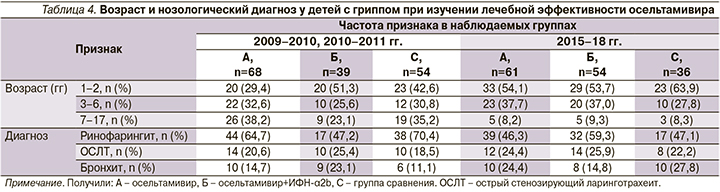
Эти наблюдения осуществлялись в основном в эпидемические подъемы гриппа. В состав сравниваемых групп вошли 312 детей в возрасте от 1 до 17 лет с верифицированным гриппом. После рандомизации 129 пациентов получили осельтамивир, 93 – сочетание осельтамивира с рекомбинантным ИФН-α2b, вводимым интраназально, 90 детей, вошедших в состав группы сравнения, патогенетически направленную базисную терапию, которую получали и пациенты остальных групп. Преобладали дети первых 6 лет жизни с гриппом типа А(H1N1)pdm, поскольку этот контингент доминировал при госпитализации как в начале, так и в конце исследований.
Клинические проявления заболеваний, имевших преимущественно среднетяжелую степень тяжести течения, зависели от возраста детей и нозологического диагноза. Но во всех случаях доминировали признаки грппа. В 100% случаев имело место острое начало с повышением температуры тела ≥38°С на фоне других проявлений интоксикации (слабость, недомогание, адинамия, снижение аппетита, у школьников – головная боль). У пяти детей регистрировали носовые кровотечения. Катаральные проявления в носоглотке детей были представлены в основном симптомами ринита и фарингита (табл. 5). У каждого четвертого имелись симптомы ОСЛТ (сухой навязчивый болезненный, часто лающий кашель, осиплость голоса). Этиотропные препараты назначали сразу после поступления ребенка в стационар.
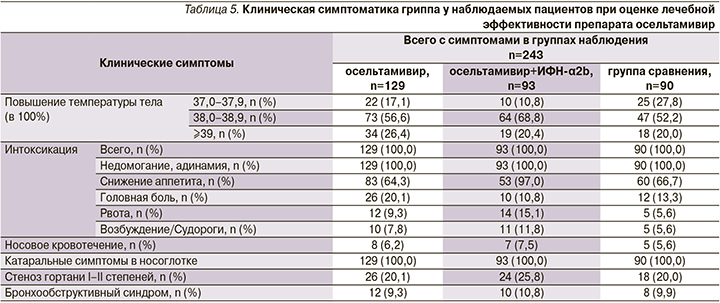
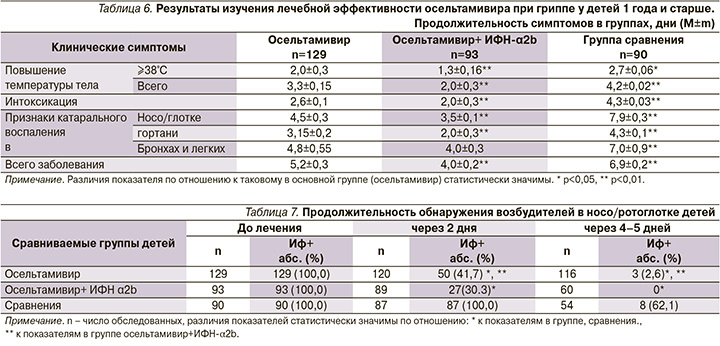
Включение препарата в терапию детей способствовало более быстрому выздоровлению. Имело место статистически значимо доказанное положительное влияние препарата на продолжительность как температурной реакции (максимальной и общей) и интоксикации, так и признаков катарального воспаления в носо/ротоглотке и гортани, а также общей продолжительности заболевания.
При этом более выраженный результат получен при введении осельтамивира в комплексе с ИФН-α2b (табл. 6).
Кроме того, показано статистически значимо более выраженное влияние осельтамивира, особенно при сочетании с интраназальным введением рекомбинантного ИФН-α2b, на сокращение вирусовыделения возбудителя гриппа в носо/ротоглотке пациентов (табл. 7).
Ни в одном из случаев не развивалось каких-либо побочных эффектов на введение препарата, даже среди детей с проявлениями дермато- и респираторного аллергозов, что подтверждалось не только клинически, но и динамикой лабораторных показателей.
Заключение
Таким образом, проведенное многоэтапное клинико-лабораторное исследование эффективности и безопасности ингибитора нейраминидазы осельтамивира при лечении детей в возрасте 1 года и старше показало возможность использования данного препарата при гриппе, подъем заболеваемости которого неминуемо будет в эпидемический сезон 2021–2022 гг. и в последующем. Оптимально сочетанное применение с рекомбинантным ИФН-α2b, который вводят интраназально.
Включение этого комплекса на ранних сроках заболевания в терапию гриппа способствует более быстрой ликвидации основных симптомов заболевания (повышенной температуры тела, интоксикации, признаков катарального воспаления в носо/ротоглотке и гортани) и таким образом способствует более быстрому выздоровлению.



