Введение
Актуальность рассматриваемой проблемы рака предстательной железы (РПЖ) определяется его лидирующими позициями в структуре онкологической заболеваемости мочеполовой системы у мужчин, находясь на 2-м месте после рака легкого и на 3-м – в структуре смертности [1]. В мире летальность у лиц с данным заболеванием на первом году жизни составляет около 30%. Отмечено, что у 60–80% пациентов при первичном обследовании выявляются метастазы или местнораспространенные формы опухоли [2]. Таким образом, социальное значение данной патологии настолько велико, что исследования в этой области занимают одно из ведущих мест в современной клинической онкологии. Ведущая роль при выборе опций терапии для пациентов с гистологически подтвержденным РПЖ отводится группам риска [3]. Идентификация пациентов, имеющих высокий и очень высокий риск, крайне важна, т.к. для их лечения возможно использование мультимодальной терапии, ориентируясь как на местные, так и на системные факторы заболевания. Следовательно, вопрос об оптимальном методе лечения данной группы пациентов остается дискутабельным [4]. В настоящее время в клинических исследованиях большой интерес вызывает опция неоадъвантной терапии (НАТ), обеспечивая быстрое получение данных о потенциальной пользе новых препаратов и предиктивных биомаркерах.
С учетом наличия андрогенной зависимости клеток РПЖ наиболее используемым методом является проведение гормональной НАТ, однако в ходе анализа литературных данных установлено, что 15% пациентов изначально резистентны к такому лечению [5]. В то же время неоадъювантная химиотерапия с последующей радикальной простатэктомией (РПЭ) ассоциируется с более высокой безрецидивной и общей выживаемостью по сравнению с пациентами группы исключительно хирургического лечения, а также позволит улучшить результаты самой операции. С 2006 г. в ФГБУ «НМИЦ онкологии им. Н.Н. Петрова» Минздрава России проводилось рандомизированное одноцентровое исследование безопасности и эффективности химиотерапии доцетакселом перед РПЭ для больных РПЖ промежуточного и высокого рисков (11,4-летнее наблюдение) [6]. Более чем десятилетние результаты данного исследования свидетельствуют о целесообразности применения комбинированного лечения больных РПЖ группы высокого риска.
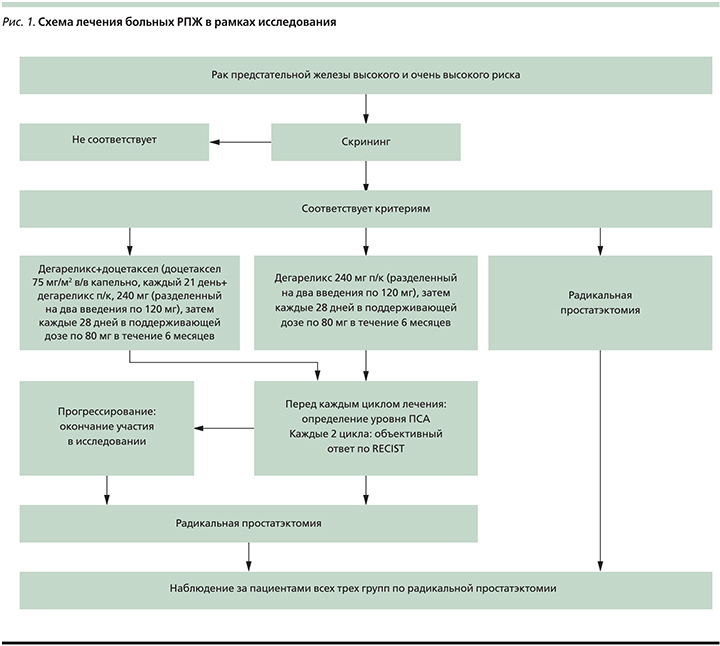
Ссуммировав вышесказанное, нами было предложено проведение клинического исследования неоадъювантного комбинированного лечения пациентов перед РПЭ при РПЖ высокого и очень высокого риска, сравнивавшего режим стандартного лечения (РПЭ) с лекарственным лечением (доцетаксел 75 мг/м2 в/в капельно каждый 21-й день+дегареликс п/к 240 мг [разделенный на два введения по 120 мг], затем каждые 28 дней в поддерживающей дозе по 80 мг) в течение 6 месяцев.
Цель исследования: повышение эффективности лечения больных РПЖ высокого и очень высокого рисков.
Методы
Материалом послужили данные о больных РПЖ высокого и очень высокого рисков, которые получали лечение в ФГБУ «НМИЦ онкологии им. Н.Н. Петрова» с 2014 по 2018 г. Все больные, включенные в исследование, имели гистологическое подтверждение аденокарциномы предстательной железы, высокий и очень высокий риски развития рецидива заболевания (ПСА >20 нг/мл и/или сумма Глисона >8, и/или клиническая стадия >T2c) и считались кандидатами для выполнения РПЭ. Ожидаемая продолжительность жизни включенных в исследование пациентов составила не менее 10 лет.
Все больные были разделены на 3 группы. На рис. 1 показана схема лечения пациентов в рамках исследования.
Статистическая обработка данных выполнена по стандартным программам для персональных ЭВМ, использованы таблицы «Excel» и стандартный пакет «Statistica 6.0» for Windows (StatSoft Inc., USA), обеспечившие выполнение общепринятых математико-статистических методов.
Результаты
В настоящей статье мы представляем промежуточные данные (от декабря 2018 г.) оценки группы исключительно комбинированного лечения: в нее включены 46 больных, 39 из которых соответствовали критериям включения и исключения. Полностью запланированный метод лечения получили 28 (61%) пациентов, остальные пациенты продолжают режим терапии.
Средний возраст мужчин, включенных в данную группу, составил 62 года (диапазон – 43–73 года).
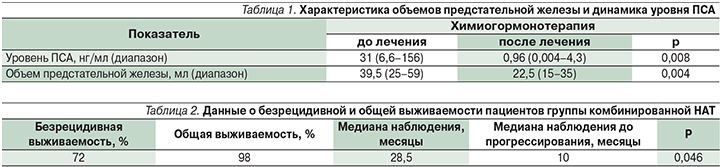
Одним из критериев эффективности проводимой НАТ служит изменение уровня ПСА (простат-специфический антиген) и объема предстательной железы. В табл. 1 представлена характеристика объемов предстательной железы и динамика уровня ПСА.
На момент проведения исследования не представлялось возможным адекватно оценить объем опухолевой ткани, поэтому эффект от проводимой НАТ основывался на определении изменений уровня ПСА и объема предстательной железы. Динамика уровня ПСА представлена на рис. 2.

Стоит отметить, что у 5 пациентов завершение этапа НАТ не представлялось возможным: у двух пациентов был отмечен рост ПСА после его предшествовавшего выраженного снижения, что свидетельствовало о прогрессировании заболевания; у двух больных терапия была прекращена вследствие негематологических серьезных нежелательных явлений (периферическая нейропатия – 1; герпетическая инфекция – 1); один пациент погиб (по причине, не связанной с проводимым лечением), остальные 74% больных (n=34) находятся под динамическим наблюдением или на различных стадиях лечения заболевания, в т.ч. рецидива. В ходе периода наблюдения за пациентами, окончившими лечение, адъювантная терапия назначена одному пациенту по результатам анализа ПСА после операции, в то время как отсроченное лечение получили 11 (28%) пациентов. Медиана наблюдения составила 28,5 месяцев. Безрецидивная выживаемость оценивалась на основании развития биохимического рецидива (БХР) заболевания. БХР расценивался как повышение ПСА более 0,2 нг/мл в двух последовательных определениях. Во всех случаях биохимический рецидив был зафиксирован раньше, чем развитие клинических симптомов. Данные представлены в табл. 2.
При изучении безопасности предложенной нами терапии выявлен приемлемый профиль токсичности, которая оценивалась после каждого введения доцетаксела и дегареликса. Высокой степени токсичности с летальным исходом не наблюдалось. Частота гематологических осложнений 3–4-й степени составила 21% (n=6). Негематологические нежелательные явления развились у 43% (n=12) пациентов, из них 3–4-й степени – 5% (n=2). У 10% пациентов отмечалась локальная болезненность в месте введения гормонального препарата. Нежелательные явления 1–2-й степеней наблюдались в 40% случаев, купировались симптоматической терапией, что не повлияло на проводимую лечебную тактику.
Обсуждение
В настоящее время, согласно актуальным клиническим рекомендациям, одной из опций выбора лечения мужчин, страдающих РПЖ высокого риска, является РПЭ [7, 8], ключевым аспектом которой сдужит радикальность лечения, проявляющаяся максимально возможным устранением опухолевого процесса. Имеются данные исследований, согласно которым хирургическое вмешательство является методом выбора для данной группы пациентов, обеспечивая хорошие онкологические результаты [9, 10]. Однако при анализе данных литературы отмечен факт наличия рецидивов заболевания более чем у 50% пациентов в течение 10 лет после проведения РПЭ локализованного РПЖ высокого риска [11]. Выбор лечебной тактики с точки зрения максимальной эффективности при минимальных осложнениях остается вопросом спорным.
Необходимо также отметить, что существенное влияние на исходы терапии оказывает количество предоперационных факторов высокого риска РПЖ [12]. Так, наличие более 2 факторов значительно ухудшает прогноз после оперативного лечения, а значит, таким пациентам нужен системный подход, сочетающий РПЭ с проведением лекарственной или лучевой терапии [13, 14].
В данной статье нами продемонстрирован промежуточный анализ ранних онкологических результатов исследования НАТ, которые позволяют предположить эффективность комбинированного лечения у ряда больных РПЖ. Отметим достоверное снижение объема предстательной железы и уровня ПСА при проведении химиогормонотерапии. Нами также отмечен приемлемый профиль переносимости и токсичности проведенного лечения.
Заключение
В результате применения НАТ в группе комбинированной терапии достигнуто статистически значимое (р=0,004) уменьшение объема предстательной железы и уровня ПСА (р=0,008). Проведение НАТ с использованием доцетаксела и дегареликса ассоциируется с улучшением результатов лечения РПЖ у больных с высоким и очень высоким риском. Применяемый нами режим терапии показал приемлемый профиль переносимости и токсичности. С учетом проспективного характера исследования планируется его продолжение до решения поставленных задач (изучение лечебного патоморфоза, молекулярно-генетических маркеров).



