Актуальность
Одной из актуальных проблем в лечении метастатических форм злокачественных опухолей является своевременное и эффективное лечение поражения костей. Наиболее часто кости скелета поражаются метастазами при раке молочной железы и раке предстательной железы (65–75%), несколько реже – при раке щитовидной железы (60%), раке легкого (40%) и раке предстательной железы (20–25%) [1].
Продолжительность жизни онкологических больных определяется наличием органных метастазов и чувствительностью опухоли к специфическому противоопухолевому лечению. Вместе с тем клинические проявления метастатической костной болезни – болевой синдром, нарушение опорной функции, патологические переломы, различные неврологические нарушения, гиперкальциемия – отягощают состояние пациентов и существенно снижают качество жизни онкологических больных.
Для оценки эффективности лечения метастазов в кости используется такое понятие, как скелетные осложнения, иначе – костные события.
К ним относят патологические переломы костей, компрессионные переломы тел позвонков (со снижением высоты тела позвонка на 25% и более между двумя рентгенологическими исследованиями), компрессию спинного мозга, появление или усиление болей, связанных с метастазами в коcти, необходимость в лучевой терапии, необходимость в хирургическом вмешательстве по поводу метастазов в кости и гиперкальциемию. Частота указанных осложнений варьируется в зависимости от характера опухоли и интенсивности проводимого лечения. Наиболее часто в течение 2 лет костные события регистрируются при раке молочной железы (64%), реже – при раке предстательной железы (49%), при раке легкого и других злокачественных опухолях (46%) [2–5].
Наиболее высок риск костных событий у пациентов с остеолитическими метастазами. С экономической точки зрения предотвращение развития костных осложнений, снижение их частоты и увеличение времени до их развития представляются, очевидно, более целесообразным, чем лечение костных осложнений [ 6].
Взаимодействие остеокластов и остеобластов в норме регулирует ремоделирование костной ткани, но опухолевые клетки нарушают этот баланс. Они производят факторы, которые стимулируют резорбцию костной ткани остеокластами и влияют на активность остеобластов путем стимулирования (при остеобластных очагах) или ингибирования (при остеолитических очагах) образования костной ткани. Выделяемые во время резорбции костной ткани ростовые факторы в свою очередь стимулируют рост опухолевых клеток [7].
Современное лечение метастазов в кости немыслимо без применения остеомодифицирующих агентов (ОМА).
Первый класс ОМА, вошедший в клиническую практику, – бисфосфонаты. По своей химической структуре они являются аналогом пирофосфатов костного матрикса, устойчивых к расщепляющему действию щелочной фосфатазы. Бисфосфонаты связываются с кальцием и избирательно накапливаются в костях. В костной ткани они утилизируются зрелыми остеокластами, угнетая их активность, действуют только в зоне костной резорбции и только после поглощения остеокластами, оказывая на них цитостатическое действие, и могут сохраняться в костной ткани до 10 лет. Бисфосфонаты первых поколений (клодроновая кислота, памидроновая и алендроновая кислоты) достаточно токсичны и неудобны в применении [8]. Большинство практических онкологов отдают предпочтение золедроновой кислоте – бисфосфонату 3-го поколения, которая более избирательно действует на костные метастазы и обладает помимо этого антиангиогенным действием и способностью вызывать апоптоз опухолевых клеток [9–11].

Первый представитель нового класса остеомодификаторов – блокатор RANK (Receptor Activator of Nuclear Factor NF-κB)-лиганда деносумаб. RANK-лиганд – основной медиатор в порочном круге костной деструкции у пациентов с солидными опухолями и метастазами в кости.
Деносумаб – таргетный препарат, который представляет собой полностью гуманизированное моноклональное антитело к RANK-лиганду, он прерывает патогенетические механизмы развития и прогрессирования костных метастазов [7, 12–14].
В отличие от бисфосфонатов, деносумаб не включается в костный матрикс, не повреждает остеокласты, а уменьшает их число, действует во внеклеточном пространстве. Это обусловливает быстрое исчезновение антирезорбтивного действия после отмены препарата [15, 16].
Комплексный анализ 3 рандомизированных двойных слепых плацебо-контролируемых исследований 3-й фазы с активным контролем по сравнению эффективности золедроновой кислоты и деносумаба, который объединил более 5000 пациентов с костными метастазами солидных опухолей, показал, что деносумаб достоверно увеличил время до возникновения первого костного события на 8,2 месяца (с 19,4 до 27,6 месяца) и снизил риск костного события на 17% (отношение рисков [ОР]=0,83, 95% доверительный интервал [ДИ] – 0,76–0,90; р<0,0001). Препарат был одинаково эффективен независимо от наличия или отсутствия костных осложнения в анамнезе [17].
Кроме того, деносумаб достоверно уменьшал уровень маркеров костной резорбции (N-телопептида мочи и остеоспецифической щелочной фосфатазы), приводил к отсрочке прогрессирования боли почти на 2 месяца, меньшему числу пациентов требовался перевод с неопиоидных анальгетиков на наркотические[18].
Из преимуществ применения деносумаба перед золедроновой кислотой следует отметить отсутствие негативного влияния на функцию почек, поэтому не требовалось мониторинга почечных функций и коррекции дозы препарата, а также удобство введения (1 подкожная инъекция в месяц). Частота возникновения остеонекроза челюсти одинакова для обоих препаратов [19].
Таким образом, при выборе остео-модифицирующего агента следует рассматривать назначение деносумаба в первую очередь пациентам с множественными костными метастазами, при высоком риске развития патологического перелома, при преимущественном поражении костей осевого скелета, при наличии болевого синдрома и исчерпанной возможности лучевой терапии, при нарушении функции почек, также при отсутствии венозного доступа.
Цель исследования: клиническая оценка эффективности и безопасности применения деносумаба при костных метастазах солидных опухолей.
Методы
Нами проанализированы данные 28 пациентов, получавших лечение деносумабом по поводу метастатического поражения костей при солидных опухолях в Ростовском научно-исследовательском онкологическом институте в 2015–2017 гг. Все пациенты имели патоморфологически подтвержденное метастатическое злокачественное новообразование (рак молочной железы, рак предстательной железы и другие опухоли) с наличием одного и более метастазов в кости. Критериями отбора для терапии деносумабом были возраст пациентов 18 лет и старше, функциональный статус по шкале ECOG (Eastern Cooperative Oncology Group)≥2. Допускалась предшествующая терапия бисфосфонатами, а также лучевая терапия или радиофармтерапия. Обязательным критерием было подписанное информированное согласие на лечение.
Деносумаб вводился подкожно в дозе 120 мг 1 раз в 28 дней. Терапия деносумабом проводилась в монорежиме либо сочеталась с химиотерапией или гормональной терапией по показаниям, согласно локализации первичной опухоли. При прогрессировании процесса пациенты продолжали получать деносумаб в сочетании с противоопухолевой терапией. До лечения и каждые 2–3 месяца от начала лечения деносумабом больным выполняли исследование костей (остеосцинтиграфия, спиральная компьютерная томография или рентгенография).
Оценивали время до развития первого костного события, частоту возникновения костных событий, динамику болевого синдрома (по визуальной аналоговой шкале – ВАШ).
Статистическая обработка данных осуществлялась с помощью пакета программ Statistica 6.0. При изучении времени до развития первого костного события использовали метод Kaplan–Meier.
В анализируемую группу вошли 28 пациентов в возрасте от 28 лет до 71 года, средний возраст составил 58,4±2,4 года, были включены 6 (21,4%) мужчин, 22 (78,6%) женщины.
Большинство больных имели метастатический рак молочной железы – 19 (67,8%), также в группу вошли пациенты раком легкого – 5 (17,8%), колоректальным раком – 2 (7,3%), раком желудка – 1 (3,6%) и раком предстательной железы – 1 (3,6%).
Все пациенты имели множественные метастазы в кости, более чем в половине случаев сопровождавшиеся выраженным болевым синдромом и ограничением объема движений (табл. 1).
С целью купирования боли всем больным применяли ненаркотические аналгетики.
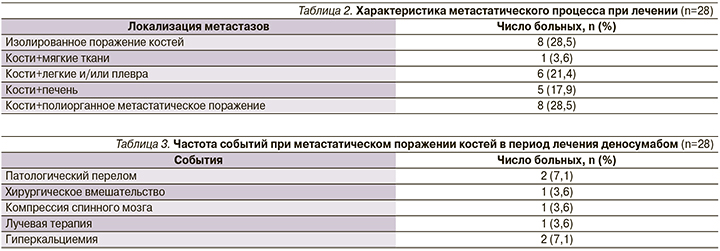
У большинства больных наличие костных метастазов сочеталось с поражением мягких тканей и внутренних органов (табл. 2).

У 8 (28,5%) больных были изолированные метастазы в кости, у 1 (3,6%) – в кости и мягкие ткани, 19 (67,9%) пациентов имели висцеральные метастазы.
У 17 (60,7%) пациентов терапия деносумабом сочеталась с химиотерапией, у 9 (32,1%) – с гормональной терапией по показаниям, согласно локализации первичной опухоли, 2 (7,3%) пациента получали только деносумаб.
В 1-й линии лечения получали деносумаб по поводу метастазов в кости 6 (21,5%) пациентов, 22 (78,5%) больных получали ранее терапию бисфосфонатами, 7 (24,9%) – лучевую и/или радиофармтерапию.
Результаты
У 22 (78,6%) больных за время терапии деносумабом отмечены репаративные изменения в очагах остеолиза по данным остеосцинтиграфии и рентгенологического обследования, у 6 (21,4%) больных деструктивные изменения в костях сохранялись на прежнем уровне, что расценено как стабилизация процесса. Нарастания степени метастатического поражения костей за время терапии деносумабом отмечено не было.
Общая частота событий при метастатическом поражении костей в период лечения деносумабом составила 7 (25%), что согласуется с литературными данными (табл. 3).
У 10 (35,7%) больных отмечалось существенное уменьшение болевого синдрома, увеличение объема движений, и они частично отказывались от приема анальгетиков после первого введения деносумаба.
К 6-му месяцу терапии выраженность болевого синдрома, оцененная по шкале ВАШ, уменьшилась на 50%, а к 12-му месяцу – на 77%.
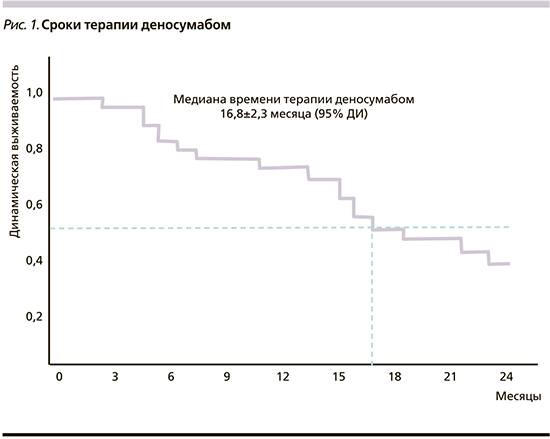
Время терапии деносумабом составило от 3 до 24 месяцев, медиана – 16,8±2,3 месяца (рис. 1). Время до возникновения первого костного события на фоне терапии деносумабом составило от 8 до 18 месяцев, медиана не достигнута (рис. 2).
Следует отметить хорошую переносимость и благоприятный профиль токсичности препарата. У 2 (7,1%)
больных отмечалась местная реакция в виде гиперемии и локальная болезненность в первые сутки после введения препарата, купировавшаяся самостоятельно. В 2 (7,1%) случаях был отмечен гриппоподобный синдром 1 ст. У 1 пациентки, изначально имевшей сопутствующую стоматологическую патологию, после 13 месяцев терапии развился остеонекроз нижней челюсти, связь которого с деносумабом нельзя исключить. Других побочных эффектов на фоне лечения данным остеомодификатором отмечено не было.
Обсуждение
Таким образом, деносумаб продемонстрировал высокую клиническую эффективность при метастазах солидных опухолей в кости. Медиана времени терапии деносумабом составила 16,8±2,3 месяца, а до возникновения первого костного события – не достигнута. Продолжительность периода наблюдения и частота возникновения костных событий сопоставимы с результатами международных исследований. Токсические явления, отмеченные на фоне терапии деносумабом, были минимальными и не повлияли на проведение противоопухолевой и остеомодифицирующей терапии.
Заключение
Таким образом, терапия деносумабом характеризуется высокой эффективностью без клинически значимых проявлений токсичности при длительном применении у больных солидными опухолями с метастазами в кости.



