Инфекция Helicobacter pylori (H. pylori) является ведущей причиной развития ряда заболеваний гастродуоденальной зоны, включая хронический гастрит, язвенную болезнь желудка и двенадцатиперстной кишки, MALT-лимфому, а также аденокарциному желудка [1, 2]. Эрадикационная терапия (ЭТ) – основной метод лечения и/или профилактики вышеперечисленных заболеваний, что отражено в ряде международных консенсусов [1, 3, 4].
На настоящий момент существует масса протоколов ЭТ, обязательными компонентами которых являются антибактериальные препараты и ингибиторы протонной помпы (ИПП) (табл. 1) [2, 4].
Вместе с тем вне зависимости от назначаемой схемы ЭТ приоритетной задачей клинициста с целью обеспечения оптимального уровня эффективности лечения конкретного больного является достижение высокой приверженности лечению [5]. Основной инструмент повышения приверженности пациента, доступный любому практическому врачу, – это беседа с подробным обсуждением основных проблем H. pylori-ассоциированных заболеваний, рисков, связанных с длительным персистированием этой инфекции в организме человека, потенциальных отрицательных сторон ЭТ, включая развитие побочных явления (ПЯ) на фоне лечения [5, 6]. Лечащему врачу важно предусматривать вероятность развития наиболее часто встречающихся ПЯ и заранее информировать о них пациента, ведь их формирование служит основной причиной снижения приверженности пациента лечению [6, 7]. Актуальность этой проблемы определена не только тем, что все схемы ЭТ первой линии включают сразу два антибактериальных препарата в высоких дозах, но и длительностью самого курса лечения, который, согласно рекомендациям консенсуса Маастрихт-V (2015), Торонтского консенсуса (2016) и рекомендациям Американской коллегии гастроэнтерологов (2017), должен быть пролонгирован до 14 дней, если отсутствуют локальные данные о приемлемой эффективности более коротких курсов терапии [8–10].
Действительно, при применении стандартных схем ЭТ ПЯ развиваются в высоком проценте случаев и могут достигать 50% [11]. Как правило, данные ПЯ не носят серьезного характера, однако в 3–10% случаев требуют отмены назначенной терапии в связи с ее непереносимостью пациентами [12]. При этом риск развития ПЯ коррелирует с длительностью курса лечения [2, 13]. Некорректная дозировка антибактериальных препаратов в схемах ЭТ также является значимым фактором риска развития ПЯ [1]. Недавно крупный мета-анализ, проведенный B.Z. Li и соавт. (2015), продемонстрировал, что риск развития ПЯ при применении ЭТ варьируется от 14 до 34%, составив в среднем 24% [14]. Наименьшая частота развития ПЯ отмечена при применении 7-дневной тройной терапии с левофлоксацином и 7-дневной тройной терапии с добавлением пробиотика. В свою очередь схемой с наименее благоприятным профилем безопасности оказались 14-дневная гибридная и 10- и 14-дневная одновременная терапия [14].
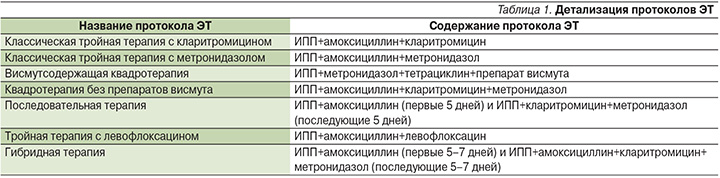
Каждый из антибактериальных препаратов обладает индивидуальным профилем безопасности и потенциальным риском развития ПЯ, спектр которых крайне гетерогенен (табл. 2) [15–17].
Так, при применении кларитромицина наиболее часто (≥10%) возможны умеренные диспепсические расстройства, тошнота, дисгевзия (нарушение вкусового восприятия). При приеме амоксициллина ПЯ встречаются несколько реже и чаще всего представлены нарушениями со стороны желудочно-кишечного тракта (ЖКТ; диарея, тошнота) и сыпью с преимущественной локализацией на конечностях и лице [16]. Крайне редко возможно развитие аллергических реакций вплоть до анафилактического шока и отека Квинке. Для метронидазола наиболее частыми (≥10%) ПЯ являются головная боль, тошнота и вагинит [17]. Несколько реже (1–10%) при приеме этого препарата развиваются металлический привкус/сухость во рту и диарея. Самым частым ПЯ при применении тетрациклина является дисколорация зубов (желтое или серо-коричневое окрашивание). Левофлоксацин обладает хорошим профилем безопасности по сравнению с другими антибактериальными препаратами, входящими в схемы ЭТ, даже при длительных курсах терапии. Тем не менее при применении данного препарата возможно развитие явлений диспепсии, головной боли, головокружения, а также инсомнии [16].
Актуальная составляющая безопасности ЭТ – это ее потенциальная токсичность. На настоящий момент антибактериальные препараты рассматриваются как довольно распространенная причина лекарственно-индуцированных поражений печени [1, 18]. Показана четкая корреляция последних с высокими дозами, полипрагмазией (в т.ч. и количеством антибиотиков в схеме терапии) и наличием сопутствующей патологии печени [1, 19, 20].
Достоверных статистических данных, позволяющих провести сравнительный анализ гепатотоксичности различных схем ЭТ, на настоящем этапе исследования проблемы, к сожалению, не получено. Согласно исследованиям последних лет, частота развития клинически релевантных антибиотико-индуцированных поражений печени при применении антибиотиков, входящих в состав схем ЭТ (амоксициллина, кларитромицина и тетрациклина), варьируется от 0,1 до 3,7 на 100 тыс. назначений [20]. В одном из исследований типа случай–контроль был продемонстрирован высокий риск развития острых антибиотикоиндуцированных поражений печени при применении макролидов (скорректированное отношение шансов [ОШ]=6,9) и тетрациклинов (скорректированное ОШ=6,2) [21]. В свою очередь субклиническое повышение уровня печеночных трансаминаз при применении макролидов наблюдается более чем в 15% случаев [22].
Отдельно стоит отметить, что сопутствующее хроническое поражение печени служит фактором, увеличивающим риск побочных лекарственных эффектов при проведении ЭТ [1, 23]. В одном из исследований показано, что частота развития ПЯ при проведении ЭТ оказалась выше у пациентов с сопутствующим хроническим гепатитом С (ОШ=1,40, 95% доверительный интервал [ДИ] – 0,56–3,44).
В этой группе пациентов отмечена более частая кумулятивная частота ПЯ (28 против 18) вне зависимости от применявшейся схемы ЭТ (тройная или последовательная) [24].
 В наших работах показано, что наличие фиброза печени вне зависимости от его выраженности (F1–F4) достоверно детерминирует риск развития ПЯ с ОШ=3,33 (95% ДИ – 1,19–9,31; p=0,0217) [15, 26]. Максимальный риск ПЯ выявлен у пациентов с циррозом печени с ОШ=4,87 (95% ДИ – 1,01–23,5; p=0,0492) [26]. Действительно, согласно литературным данным, пациенты с циррозом печени относятся к достоверно высокому риску развития ПЯ ввиду снижения метаболизма и клиренса лекарственных веществ, а также более высокой вероятности негативных лекарственных взаимодействий [27–29]. При этом антибактериальные препараты занимают третье место в структуре развития ПЯ у пациентов этой категории [29].
В наших работах показано, что наличие фиброза печени вне зависимости от его выраженности (F1–F4) достоверно детерминирует риск развития ПЯ с ОШ=3,33 (95% ДИ – 1,19–9,31; p=0,0217) [15, 26]. Максимальный риск ПЯ выявлен у пациентов с циррозом печени с ОШ=4,87 (95% ДИ – 1,01–23,5; p=0,0492) [26]. Действительно, согласно литературным данным, пациенты с циррозом печени относятся к достоверно высокому риску развития ПЯ ввиду снижения метаболизма и клиренса лекарственных веществ, а также более высокой вероятности негативных лекарственных взаимодействий [27–29]. При этом антибактериальные препараты занимают третье место в структуре развития ПЯ у пациентов этой категории [29].
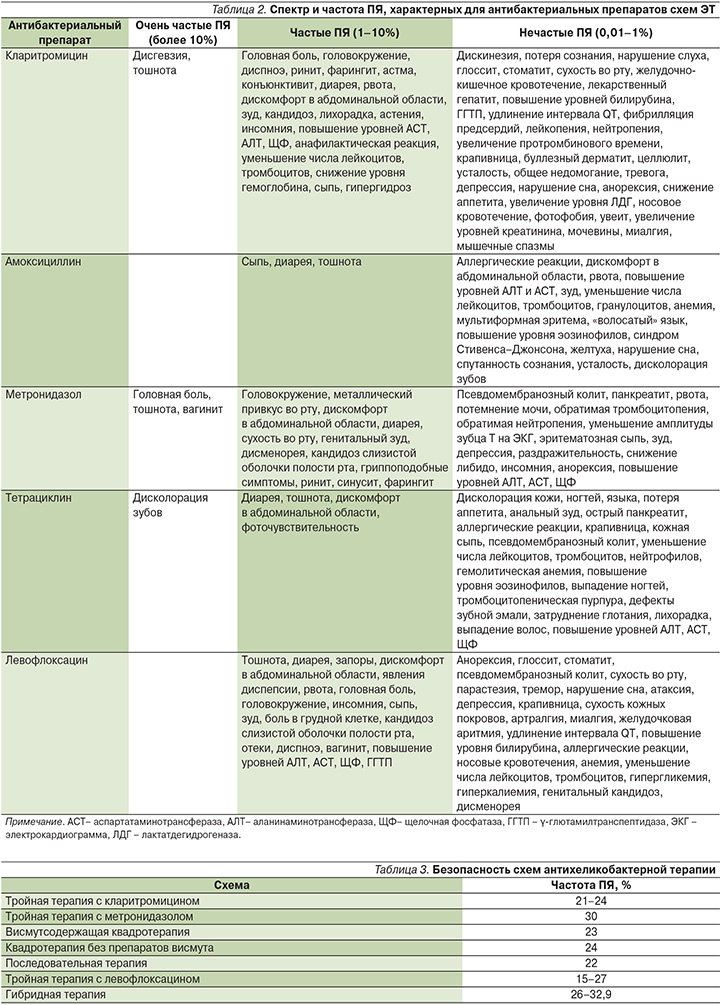
Как уже говорилось выше, все существующие схемы ЭТ предполагают назначение сразу нескольких антибактериальных препаратов, что детерминирует различный риск развития ПЯ при использовании конкретной схемы лечения (табл. 3).
Так, частота ПЯ на фоне применения тройной терапии с кларитромицином достигает 24% при 14-дневных курсах терапии [14]. Наиболее частыми ПЯ, развивающимися у пациентов: дисгевзия (нарушение восприятия вкуса), тошнота и диарея [31]. ПЯ при применении тройной терапии с метронидазолом развиваются у 30% больных [30]. При этом выраженные ПЯ, требующие прекращения курса ЭТ, наблюдаются лишь у 1,4% пациентов [31]. Частота ПЯ на фоне применения 10- и 14-дневных курсов висмутсодержащей квадротерапии составляет 23% [14]. Использование этой схемы ЭТ влечет за собой риск развития таких ПЯ, как головная боль, тошнота, дисколорация зубов и металлический привкус во рту [31]. Использование квадротерапии без препаратов висмута, как правило, характеризуется умеренной частотой развития ПЯ, составляющей в среднем 24% [14]. У пациентов могут наблюдаться тошнота, дисгевзия, металлический привкус во рту, сухость во рту, диарея, головная боль и повышение уровней АСТ и АЛТ в сыворотке крови [31]. При применении последовательной терапии риск развития ПЯ, несмотря на наличие трех антибактериальных препаратов в ее составе, несколько ниже по сравнению с классическими схемами, т.к. каждый из этих препаратов применяется не более 5 дней. Общая частота ПЯ составляет 22% [14]. При этом к наиболее частым ПЯ при применении данной схемы можно отнести дисгевзию, металлический привкус во рту, тошноту и головную боль. Применение тройной терапии с левофлоксацином, как правило, характеризуется хорошим профилем безопасности. При 7-дневных курсах терапии ПЯ развиваются лишь у 15% пациентов [14]. К наиболее частым ПЯ при применении данной схемы ЭТ можно отнести тошноту, диарею, головную боль, инсомнию, а также повышение уровней АСТ и АЛТ в сыворотке крови. Несмотря на высокую эффективность, гибридная терапия отличается субоптимальным профилем безопасности. Частота ПЯ при применении этой схемы ЭТ варьируется от 26 до 32,9%, находясь в зависимости от длительности курса лечения [14, 32–34]. Тем не менее лишь в 2,5% случаев требуется прекращение курса ЭТ в связи с развитием выраженных ПЯ [34]. Как правило, у пациентов отмечаются тошнота, дисгевзия, металлический привкус или сухость во рту, а также диарея [31].
Достоверное снижение частоты ПЯ на фоне проводимой ЭТ достигается только с использованием адъювантной терапии с применением пробиотиков [5]. Действительно, на сегодняшний день сразу несколько мета-анализов демонстрируют, что добавление пробиотиков на основе Saccharomyces boulardii, Lactobacillus spp., а также Bifidobacterium lactis и Bifidobacterium bifidum в стандартные схемы ЭТ увеличивает частоту эрадикации на 8,1–14,1%, а также снижает частоту ПЯ, связанных с ЭТ, особенно диарею и нарушения вкусового восприятия [35–39]. Один из недавних мета-анализов, объединивший результаты 21 исследования, наглядно продемонстрировал снижение риска ПЯ при использовании ЭТ в сочетании с пробиотиками с отношением рисков [ОР]=0,60 (95% ДИ – 0,40–0,91) [38]. Последний мета-анализ 29 исследований (3122 пациента) также продемонстрировал, что адъювантная терапия с применением пробиотиков достоверно снижает частоту ПЯ ЭТ с ОР=0,49 (95% ДИ – 0,38–0,65) [39].
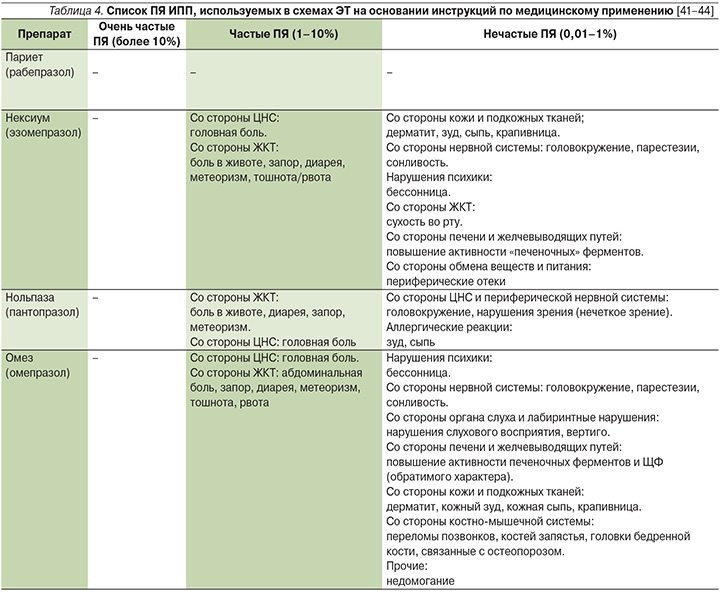
Помимо антибактериальных препаратов все схемы ЭТ содержат ИПП, которые индивидуально отличаются хорошим профилем безопасности. При коротких курсах терапии описаны ПЯ со стороны центральной нервной системы (ЦНС), такие как повышенная утомляемость (2%), головная боль (2–3%), головокружение (1%), и со стороны ЖКТ (диарея у 2% больных или запоры у 1% пациентов). В редких случаях отмечаются аллергические реакции, которые клинически проявляются кожной сыпью или явлениями бронхоспазма (табл. 4) [40].
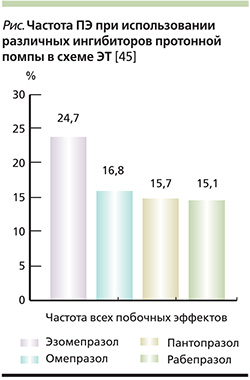
Тем не менее потенциальный риск развития ПЯ при использовании различных ИПП в схемах ЭТ различен, что может быть обусловлено конкурентным взаимодействием с антибактериальными препаратами на фармакокинетическом уровне. Так, в исследовании H.S. Choi и соавт. (2007) выявлено, что частота ПЯ при использовании различных ИПП в схеме ЭТ неоднородна с большим превалированием в группе эзомепразола (24,7%) и минимальным в группе Париета (рабепразола) (15,1%) (см. рисунок) [45].
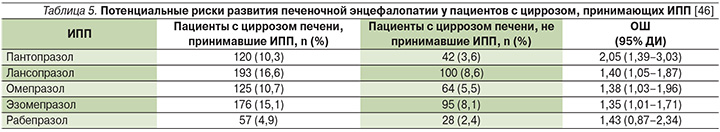
На сегодняшний день повышенное внимание уделено ПЯ, ассоциированным с длительной и непрерывной (иногда необоснованной) курсовой терапией ИПП. В частности, лечение ранними представителями ИПП (омепразол, лансопразол и пантопразол) в высоких дозах, по данным некоторых авторов, может приводить к обратимой гипергастринемии и гиперплазии ECL-клеток слизистой оболочки желудка [40]. Также хотелось бы упомянуть, что в недавнем популяционном исследовании у пациентов с циррозом печени выявлены дополнительные риски развития печеночной энцефалопатии на фоне длительного приема ИПП, кроме оригинального рабепразола (Париета) (табл. 5) [46].
Таким образом, использование рабепразола в схемах ЭТ представляется наиболее оптимальной стратегией оптимизации антисекреторного звена ЭТ, характеризующейся как минимальной частотой ПЯ, так и высокой эффективностью [5]. Как известно, скорость метаболизма, а соответственно, и эффективность ИПП в первую очередь детерминирована полиморфизмом гена, кодирующего изоформу системы цитохрома Р450 – CYP2С19 [47, 48]. В зависимости от типов мутаций CYP2С19 популяцию можно подразделить на четыре фенотипические группы: «быстрые», «промежуточные», «медленные» и «ультрабыстрые» метаболизаторы [47, 49]. Пациенты с фенотипом «быстрых» и «ультрабыстрых» метаболизаторов осуществляют ускоренный метаболизм ИПП, а следовательно, антисекреторный эффект от приема ИПП у них имеет меньшую выраженность, чем у пациентов с фенотипами «промежуточных» и «медленных» метаболизаторов [6, 47, 48]. Менее выраженный антисекреторный эффект от «быстрых» и «ультрабыстрых» метаболизаторов определяет низкую эффективность эрадикационной терапии инфекции H. pylori у лиц этих фенотипов [5, 6, 49–51]. Так, в мета-анализе S. Padol и соавт. была продемонстрирована более высокая эффективность ЭТ у пациентов с фенотипами «медленных» (88,9%) и «промежуточных» (82,7%) метаболизаторов по сравнению с «быстрыми» (70,9%) [52]. Рабепразол преимущественно метаболизируется неэнзиматическим путем, за счет чего обладает более стабильным профилем фармакокинетики (наименьший разброс показателя AUC – Area Under Curve в зависимости от генотипа), в меньшей степени зависящим от полиморфизмов CYP2С19 [5, 6, 51, 53]. Данное свойство рабепразола обеспечивает более предсказуемый и устойчивый антисекреторный эффект по сравнению с другими ИПП. Согласно результатам мета-анализа H.L. Tang и соавт., рабепразол-содержащие схемы тройной ЭТ H. pylori имеют минимальные различия в эффективности между различными генетически-детерминированными вариантами метаболизма [54]. Другой мета-анализ A.G. McNicholl и соавт., включивший 35 исследований (5998 пациентов), продемонстрировал, что использование оригинального рабепразола (Париет) в схемах тройной эрадикационной терапии определяет более высокую эффективность антихеликобактерного лечения (ОШ=1,21, 95% ДИ – 1,02–1,42) по сравнению с ИПП первых генераций (омепразол, лансопразол, пантопразол) [55]. Согласно рекомендациям Маастрихт-V (2015), рабепразол предложен в качестве ИПП, наименее подверженного влиянию генотипа CYP2C19, что обосновывает его предпочтительное использование в странах Европы и Северной Америки, где высока распространенность фенотипа «быстрых» метаболизаторов [8]. То же касается и нашей страны, где распространенность фенотипа «быстрых» метаболизаторов составляет 32,65%, а «ультрабыстрых» – 39,75% [56].
Важно отметить, что эффективность применения оригинального рабепразола (Париет) в схемах ЭТ объясняется также собственной антихеликобактерной активностью препарата, превышающей другие ИПП, в т.ч. на полирезистентные штаммы H. pylori, в существенной степени снижающей минимальные ингибирующие концентрации антибиотиков в схемах эрадикации, подавляя резистентный потенциал микроорганизма [51, 57, 58]. Дополнительное воздействие рабепразола на факторы защиты, такие как стимуляция секреции слизи и муцинов в слизистой оболочке желудка, обусловливает дополнительное преимущество его использования в схемах как ЭТ, так и терапии гастроэзофагеальной рефлюксной болезни [51, 59].
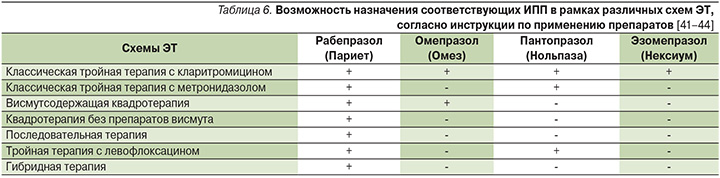
С другой стороны, текущий статус эффективности и безопасности ЭТ осложняется предписанной в инструкциях для большинства ИПП определенной схемы терапии, которая состоит из комбинации ИПП и двух четко предписанных антибиотиков и не подразумевает их замены, а также добавления потенцирующих агентов, таких как препараты висмута и пробиотики, в связи с ситуацией off-label (использование лекарственных средств по показаниям, не утвержденным государственными регулирующими органами и не упомянутым в инструкции по применению). В связи с этим фактором, особенно значимым, становится схема терапии с использованием оригинального рабепразола (Париета), которая не имеет предписаний в отношении комбинации антибиотиков, не регламентирует их вариативность, а также позволяет добавлять препараты висмута и пробиотики в любой линии терапии, что открывает возможности многогранной оптимизации ЭТ (табл. 6), которая становится все более актуальной в связи с последними данными о нарастающей антибиотикорезистентности отдельных регионов Российской Федерации [60–63].
Таким образом, риск развития ПЯ при применении ЭТ составляет в среднем 24% и варьируется в зависимости от конкретной схемы лечения: тройная терапия с кларитромицином (21–24%), тройная терапия с метронидазолом (30%), висмутсодержащая квадротерапия (23%), квадротерапия без препаратов висмута (24%), последовательная терапия (22%), тройная терапия с левофлоксацином (15–27%), гибридная терапия (2–32,9%). К факторам, повышающим риск развития ПЯ, относятся длительность курса терапии, некорректная дозировка препаратов, наличие сопутствующей патологии печени, включая цирроз. Достоверное снижение частоты ПЯ на фоне проводимой ЭТ достигается только с использованием адъювантной терапии с применением пробиотиков. Использование рабепразола (Париета) в схемах ЭТ представляется наиболее оптимальной стратегией оптимизации антисекреторного звена ЭТ, характеризующейся как минимальной частотой ПЯ, так и высокой эффективностью.



