Введение
В начале 2000-х гг. в мировой литературе появились первые публикации о влиянии циклинзависимых киназ (CDK) на пролиферацию опухолевых клеток и о возможности ингибирования CDK в рамках потенциальной противоопухолевой терапии [1].
Известно, что CDK4/6 участвуют в клеточном цикле, способствуя переходу клетки из фазы G1, фазы митоза, в синтетическую S-фазу путем образования комплекса с циклином D1 (CycD1) и фосфорилирования белка Rb (рис. 1). В опухолевых клетках CDK4/6 обладают патологической активностью и выступают в качестве драйверов клеточной пролиферации, что приводит к их бесконтрольному размножению.
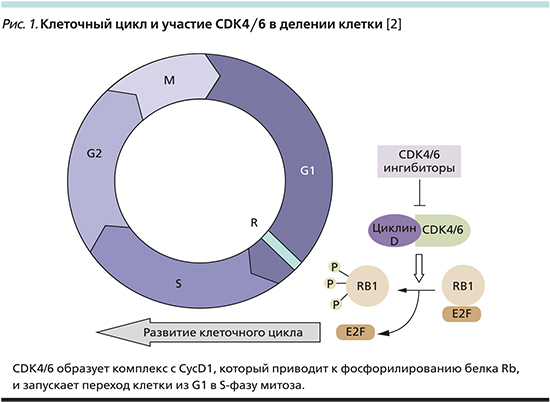
В экспериментах in vitro было показано, что эстрадиол запускает клеточную пролиферацию, воздействуя на уже обозначенные CDK и циклины. Так, в эксперименте O.W. Prall et al. клетки культуры рака молочной железы (РМЖ), обработанные антагонистами эстрогена, впадали в арест клеточного цикла на фазе G1 [3]. Таким образом, дополнительно блокируя переход из G1- в S-фазу, можно добиться дополнительного противоопухолевого эффекта. Впервые синергизм iCDK4/6 и эндокринотерапии был продемонстрирован in vitro R.S. Finn et al. на примере комбинации палбоциклиба и тамоксифена [4]. Также было показано, что нарушение регуляции комплекса CDK4/6-CycD1-Rb приводит к развитию эндокринорезистентности (ЭР) [5]. Именно поэтому ингибиторы CDK4/6 (iCDK4/6) привлекли пристальное внимание исследователей в качестве терапии люминального HER2-негативного (HR+/HER2-) РМЖ.
На сегодняшний день в Российской Федерации зарегистрировано три пероральных препарата класса iCDK4/6: рибоциклиб (цикл регистрационных исследований MON-ALEESA), палбоциклиб (цикл PALOMA) и абемациклиб (цикл MONARCH).
Согласно современным рекомендациями по лечению РМЖ, iCDK4/6 рассматриваются в качестве наиболее предпочтительной опции 1-й или 2-й линии лечения HR+/HER2-метастатического РМЖ (мРМЖ).
В 2019 г. опубликованы данные крупного мета-анализа, в котором M. Giuliano et al. проанализировали данные 140 исследований II и III фаз, сравнивавших химио- (ХТ) и эндокринотерапию в лечении HR+/HER2- мРМЖ [6]. Авторы приходят к выводу, согласно которому ни один режим ХТ не превосходит комбинацию iCDK4/6 и эндокринотерапии по показателю выживаемости без прогрессирования (ВБП) при значимо более благоприятном профиле токсичности.
Абемациклиб: уникальность молекулы
Абемациклиб – последний из зарегистрированных пероральных iCDK4/6, который обладает некоторыми фармакокинетическими особенностями, отличающими его от других представителей рассматриваемого класса [7]. Он уникален высокоселективным ингибированием комплексов CDK4/CycD1 (концентрация полумаксимального ингибирования (IC50=2 нмоль/л) и CDK6/CycD1 (IC50=10 нмоль/л), при этом не связывается с другими CDK (кроме CDK9). При этом афинность абемациклиба к CDK4 в 14 раз выше, чем к CDK6 [8]. По сравнению с другими препаратами класса селективность в отношении CDK4/CycD1 у абема-циклиба значительно выше, чем у палбоциклиба и рибоциклиба (показатель IC50 у абема-циклиба в 5 раз ниже) [9].
Помимо активности в клеточном цикле S. Goel et al. продемонстрировали иммуномодулирующие свойства абемациклиба путем потенцирования презентации опухолевых антигенов и селективной супрессии регуляторных Т-лимфоцитов. В моделях трансгенных мышей абемациклиб моделирует микроокружение опухолевых клеток, стимулирует выработку интерферонов III типа и связанных с ними транскрипционных факторов (таких, как STAT1/2, IRF2/6,9, NLRC5). Также отмечено уменьшение числа регуляторных Т-лимфоцитов, обладающих иммуносупрессивным действием, в селезенке и лимфатических узлах, при этом число цитотоксических CD8+ Т-лимфоцитов оставалось неизменным. Полученные данные могут косвенно свидетельствовать о стимуляции иммунного ответа при применении абемациклиба [10].
Применение абемациклиба в монотерапии предлеченных пациенток с HR+/HER2- мРМЖ: исследование MONARCH-1
По результатам исследований эффективности применения палбоциклиба и рибоциклиба в монотерапии предлеченных пациенток с HR+/HER2- мРМЖ, опуболикованных в 2015 и 2016 гг., ни один из изучаемых препаратов не продемонстрировал клинического преимущества [10–12].
Однако результаты исследования MONARCH-1 изменили представление о применении iCDK4/6 в монорежиме [13]. В указанном исследовании изучали применение абемациклиба в монотерапии у предлеченных пациенток с HR+/HER2- мРМЖ. Дизайн предполагал включение пациенток, получивших не менее двух линий лечения по поводу HR+/HER2- мРМЖ и имевших признаки ЭР.
В исследовании 132 пациентки получали монотерапию абемациклибом в дозе 200 мг 2 раза в сутки (каждые 12 часов). Первичной конечной точкой исследования стала оценка частоты объективного ответа (ЧОО), вторичными – общая выживаемость (ОВ), ВБП и безопасность терапии.
Как уже было отмечено, в исследование включались предлеченные пациентки: среднее число линий системной терапии составило 5 (от 2 до 11), а по поводу метастатической болезни – 3 (от 1 до 8). При этом протокол исследования допускал как применение эндокринотерапии, так и ХТ в анамнезе.
Первичная конечная точка оценивалась через 18 месяцев наблюдения. Подтвержденная ЧОО составила 19,7%, а частота клинического преимущества (ЧКП), включившая ЧОО и стабилизацию заболевания не менее 6 месяцев, достигнута 42,4% пациенток. Медиана времени достижения объективного ответа составила 3,67 месяца.
Подгрупповой анализ результатов MONARCH-1 оценил эффективность терапии пациенток различных популяций, обладавшх неблагоприятными факторами прогноза.
Среди включенных в исследование пациенток большинство (70,5%) имели метастатическое поражение печени.
В этой подгруппе (рис. 2а) ЧОО составила ~12%, ЧКП – 40%. В группе пациенток без метастатического поражения печени аналогичные показатели составили 20 и 40% соответственно [14].
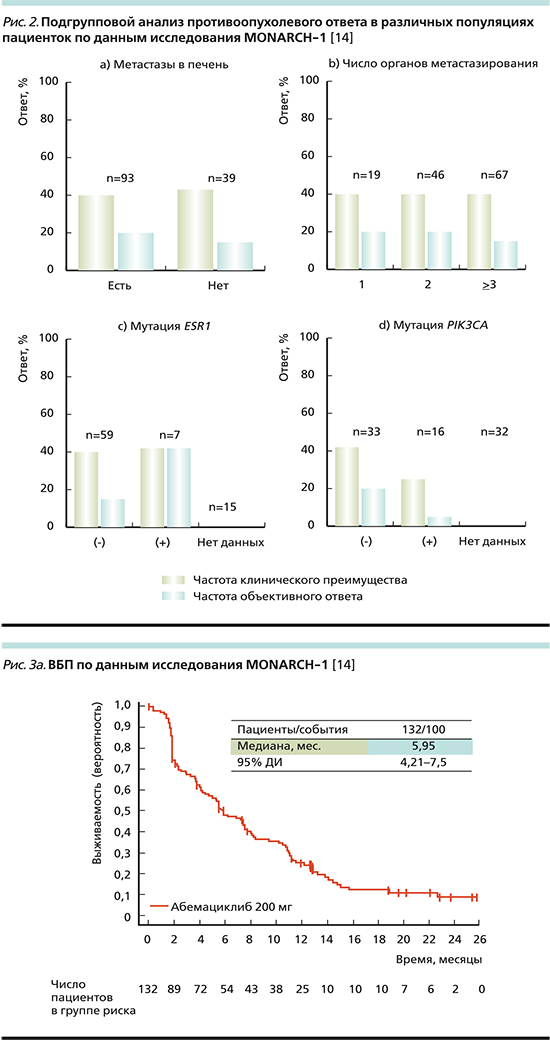
Также у 50,8% включенных пациенток была отмечена значительная опухолевая распространенность (не менее трех метастатически пораженных органов). При подгрупповом анализе (рис. 2b) отмечено, что в этой крайне неблагоприятной прогностической группе ЧОО составила 17%, ЧКП – 40% (что сопоставимо с группами пациенток, имевших 1 или 2 сайта метастазирования).
Также изучалась эффективность в подгруппе пациенток, имевших мутацию в генах ESR1 и PIK3CA. Обе мутации обусловливают развитие ЭР и являются негативными прогностическими факторами.
ЧОО при наличии мутации гена ESR1 (рис. 2с) составила 40 и 10% без нее, при этом ЧКП достигла 40% у ESR1+ и 37% у ESR1-. ЧОО у пациенток с PIK3CA+ составила 3 и 20% в группе PIK3CA- (рис. 2d). ЧКП в группе PIK3CA+ – 22%, 41% в группе PIK3CA-.
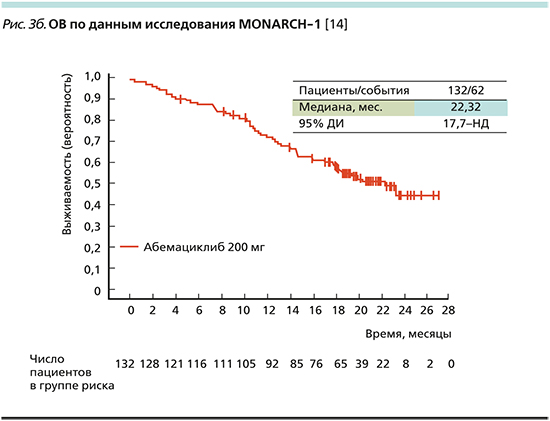
По результатам данного исследования медиана ВБП составила 5,95 месяца (рис. 3а), медиана ОВ – 22,32 месяца (рис. 3b) [14].
Наиболее частыми нежелательными явлениями (НЯ) оказались диарея (90,2% всех степеней) и нейтропения (87,7% всех случаев). Подробнее профиль безопасности и НЯ на фоне терапии абемациклибом будут рассмотрены в соответствующем разделе.
Клиническое преимущество использования абемациклиба сильно предлеченными пациентками, правда в комбинации с тамоксифеном, было продемонстрировано еще в одном исследовании – nextMONARCH.
В 2020 г. опубликованы окончательные результаты этого исследования, что еще раз показало, что и этой популяцией пациенток возможно достижение медианы ВБП 7,4 месяца, а ЧОО – 32,5% [15].
Причины, по которым именно абемациклиб достиг успеха в монотерапии, остаются не до конца изученными. Возможно, влияние оказывает режим приема препарата: абемациклиб – единственный iCDK4/6, принимаемый дважды в сутки каждые 12 часов ежедневно без перерыва, что позволяет поддерживать постоянную концентрацию препарата в плазме крови [13]. Доклинические исследования на моделях ксенотрансплантата РМЖ показали, что применение абемациклиба обеспечивает подавление роста в течение всего времени его применения, но даже кратковременное прерывание ингибирования CDK4/6 приводит к эффекту «рикошета» и может снижать противоопухолевую эффективность [16–18].
Применение абемациклиба в комбинации с фулвестрантом во 2-й линии лечения HR+/HER2- мРМЖ: исследование MONARCH-2
Исследованием, зарегистрировавшим комбинацию абемациклиба с фулвестрантом в лечении HR+/HER2- мРМЖ, стало MONARCH-2 [19]. Дизайн исследования предполагал включение пациенток в пре-, пери- и постменопаузе, которые ранее не получали ХТ по поводу мРМЖ, а также пациенток, у которых наблюдались признаки ЭР, либо пациентки имели прогрессирование болезни на фоне нео- или адъювантной эндокринотерапии, либо на фоне эндокринотерапии 1-й линии.
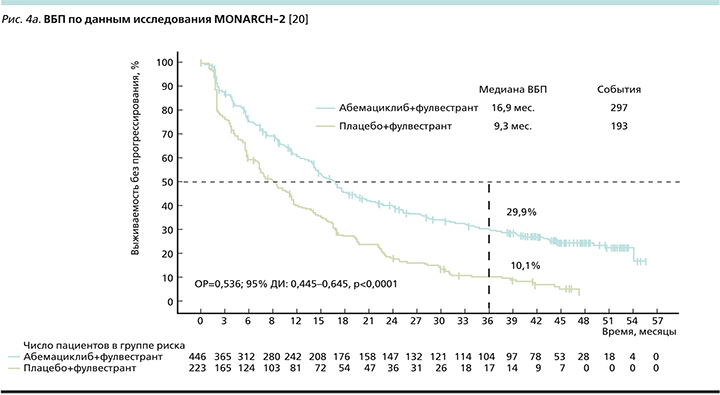
В две группы были рандомизированы 669 пациенток в соотношении 2:1 в пользу исследуемой терапии: в экспериментальной группе пациентки получали терапию абемациклиб+фулвестрант, в контрольной – только фулвестрант. Первичной конечной точкой исследования являлась оценка ВБП (рис. 4a). Согласно опубликованным данным, добавление абемациклиба к фулвестранту обеспечивает прибавку в 7,6 месяца к медиане ВБП (16,9 месяца против 9,3 месяца), снижая относительный риск прогрессирования (ОРП) на 46% [20].
Вторичной конечной точкой оценивалась ОВ (рис. 4b), медиана которой оказалась выше на 9,4 месяца больше по сравнению с контрольной группой (46,7 против 37,3 месяца), относительный риск смерти (ОРС) при добавлении абемациклиба к фулвестранту снижался на 24% [20].
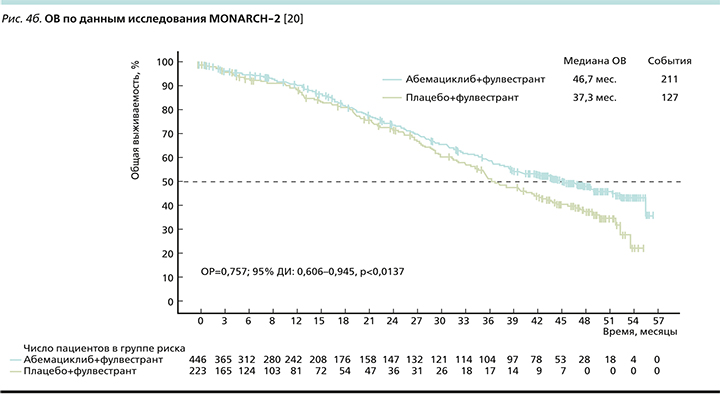
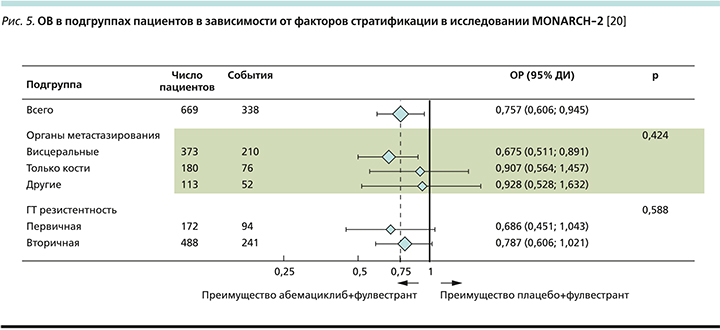
При рассмотрении подгруппового анализа (рис. 5) отмечено, что наибольший выигрыш имели пациентки с висцеральными метастазами (ОРС снижался на 33%). Также наибольшее клиническое преимущество наблюдалось в группе пациенток с эндокринорезистентными опухолями как с имеющейся первичной (ОРС снижался на 31%), так и при развитии вторичной ЭР (ОРС снижался на 22%) [20].
Поскольку одной из основных целей лечения мРМЖ является не только продление продолжительности жизни, но и улучшение ее качества, нельзя не упомянуть о таком параметре, как время до назначения ХТ. Безусловно, ХТ снижает качество жизни пациентки ввиду присущих ей НЯ, именно поэтому важно как можно дольше отсрочить момент начала ХТ. Согласно данным исследования MONARCH-2, пациентки, получавшие комбинацию абема-циклиба с фулвестрантом, перешли к химиотерапевтическому лечению на 28 месяцев позже пациенток, получавших только фулвестрант (50,2 против 22,1 месяца).
Профиль токсичности, оцененный по результатам MONARCH-2, был сходным с результатами MONARCH-1. Наиболее частыми НЯ являлись диарея (87,1% всех степеней) и нейтропения (49,7% всех случаев).
Применение абемациклиба в комбинации с ингибиторами ароматазы в 1-й линии лечения HR+/HER2- мРМЖ: исследование MONARCH-3
Исследование MONARCH-3 позволило зарегистрировать абемациклиб в комбинации с ингибиторами ароматазы (ИА) и в 1-й линии лечения HR+/HER2- мРМЖ [21]. Дизайн исследования подразумевал включение постменопаузальных пациенток, которые ранее не получали терапию по поводу распространенной болезни и не обладали признаками ЭР.
Как и в исследовании MONARCH-2, пациенток рандомизировали в две группы в соотношении 2:1 в пользу экспериментальной группы: абемациклиб+ИА против ИА в монорежиме.
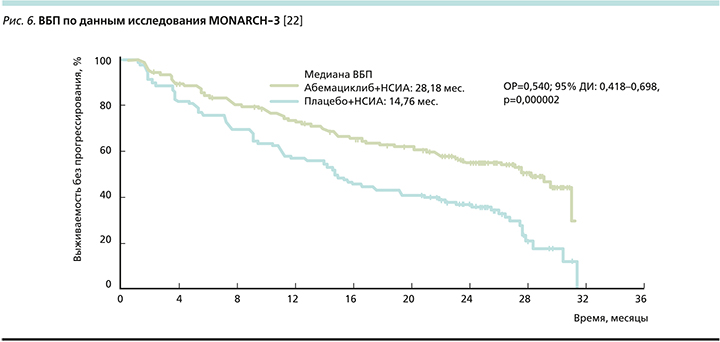
При оценке ВБП (рис. 6) отмечено, что комбинация абемациклиба и ИА добавила 13,4 месяца (28,2 против 14,8 месяца) до прогрессирования болезни, снижая ОРП на 46% по сравнению с монотерапией ИА [22].
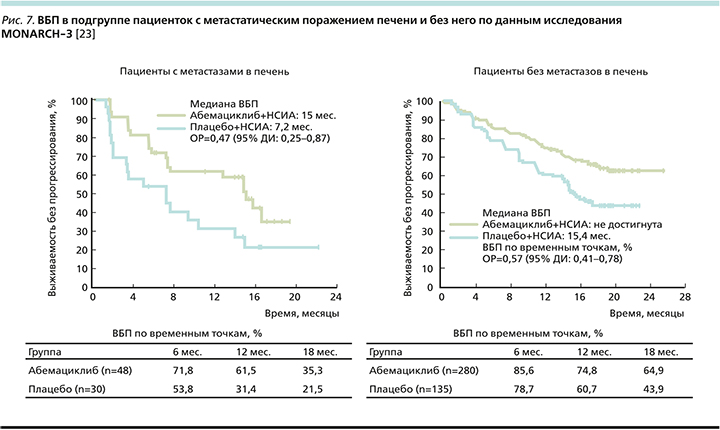
Отмечено выраженное клиническое преимущество группы пациенток с висцеральными метастазами: ОРП снижался на 39%, а при наличии метастатического поражения печени (рис. 7) ОРП снижался на 53% (в отсутствие метастазов в печени относительный риск снижался на 43%) [23].
ЧОО в общей популяции пациенток составила 49,7 против 37% в контрольной группе, при этом у 9% добавление абемациклиба позволило достичь полного ответа по RE-CIST1.1 (против 1% в группе монотерапии) [23].
Данные по ОВ в исследовании MONARCH-3 на сегодняшний день не опубликованы, поскольку на настоящее время зарегистрировано недостаточное число событий.
Профиль токсичности у пациенток, получавших абемациклиб, был схож с таковым в исследованиях MONARCH-1 и -2. Как и в первых двух исследованиях, наиболее частыми НЯ оказались диарея (82,3% всех степеней) и нейтропения (43,7% всех случаев).
Абемациклиб и метастатическое поражение головного мозга при HR+/HER2- мРМЖ
В мировой литературе накоплено некоторое количество данных, описывающих эффективность абемациклиба при интракраниальном опухолевом поражении (как первичном, так и вторичном) [24–26]. Высокая специфичность в связывании CDK4 важна с точки зрения способности молекулы проникать через гематоэнцефалический барьер. In vivo показано, что концентрация абемациклиба в спинномозговой жидкости (СМЖ) после начала терапии была сравнимой с концентрацией препарата в плазме крови [27].
Во всех регистрационных исследованиях цикла MONARCH наличие метастатического поражения центральной нервной системы (ЦНС) служило критерием исключения, поэтому объективная оценка эффективность терапии абемациклибом пациенток такой сложной подгруппы затруднена. В 2020 г. были опубликованы результаты исследования II фазы, в котором оценивалась эффективность абемациклиба в лечении метастатического поражения головного мозга при HR+/HER2- мРМЖ [28]. Исследователи использовали абемациклиб либо в монотерапии, либо в комбинации с эндокринотерапией. Также S.M. Tolaney et al. проанализировали уровень концентрации абемациклиба в СМЖ и концентрацию препарата в плазме крови. По данным авторов, интракраниальная ЧОО у пациенток с поражением ЦНС составила 5,2%, стабилизация – 18,8%. Медиана ОВ при этом достигла 12,5 месяца. Уровни концентрации абемациклиба в СМЖ и плазме крови оказались равными.
Современный стандарт лечения метастатического поражения головного мозга предполагает применение радиохирургического лечения (чаще всего стереотаксическую лучевую терапию или облучение всего головного мозга). Однако, поскольку при люминальном РМЖ изолированное интракраниальное поражение развивается редко, важно продолжать системную противоопухолевую терапию, но при приемлемой токсичности, не увеличивая ее за счет суммации лучевой и лекарственной терапии. E. Harvey-Jones et al. проанализировали клинические случаи 23 пациенток, которые одновременно получали абемациклиб и прошли курс лучевой терапии по поводу интракраниальных метастазов HR+/HER2- мРМЖ [29]. Авторы приходят к выводу, согласно которому одновременная лучевая терапия и прием абемациклиба совместимы, безопасны и не приводят к усилению токсичности.
НЯ терапии абемациклибом
Опубликованные крупные мета-анализы, а также данные регистрационных исследований позволяют судить о том, что все iCDK4/6 различаются профилем токсичности [30–32]. Так, D. Ribnikar et al. проанализировали данные 8 рандомизированных исследований, в которых изучали применение iCDK4/6 [33]. Всего в исследованиях 873 пациентки получили палбоциклиб, 1153 – рибоциклиб и 773 – абемациклиб. По результатам данного мета-анализа оказалось, что абемациклиб обладает значимо меньше выраженной гематологической токсичностью, чем любой другой iCDK4/6, однако значительно чаще вызывает диарею. Выявленные различия могут объясняться фармакокинетическими особенностями молекул препаратов.
Особенности нейтропении, вызванной абемациклибом
CDK6 регулирует состояние покоя в гемопоэтических стволовых клетках человека и служит главной CDK в кроветворных клетках-предшественниках. CDK6 действует как транскрипционный регулятор супрессии в гемопоэтических стволовых клетках, запуская их созревание в костном мозге. Именно поэтому ингибирование CDK6 характеризуется нарушениями гемопоэтической системы [34].
Напомним, что абемациклиб в меньшей степени, чем CDK4, ингибирует CDK6, а также меньше аффинен к ней, чем любой другой iCDK4/6 [35]. Вероятнее всего, этот факт объясняет меньшую частоту нейтропении на фоне терапии абемациклибом: в исследовании PALOMA-2 частота нейтропении составила 79,5%, из которых 66,4% приходятся на Gr.-3–4, а в исследовании MONARCH-3 – только 41,3% при 21,1% Gr.-3–4.
Характеристика нейтропении, развивающейся на фоне абемациклиба (по данным исследований MONARCH), отражена в табл. 1.
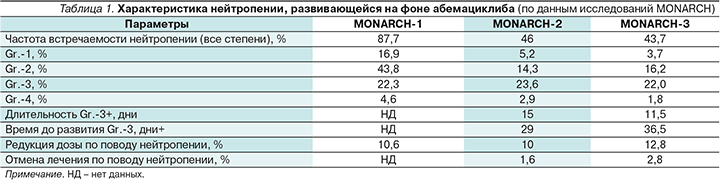
Стоит отметить, что более высокая частота встречаемости нейтропении всех степеней в исследовании MONARCH-1 по сравнению с MONARCH-2 и -3 может объясняться более высокой дозой абемациклиба: 200 мг 2 раза в сутки против 150 мг 2 раза в сутки в последующих исследованиях.
В комбинации с эндокринотерапией абемациклиб вызывал сопоставимую частоту нейтропении Gr.3: 23,6% в комбинации с фулвестрантом и 22,0% в комбинации с ИА. Частота встречаемости нейтропении Gr.-4 составила 2,9 и 1,8% соответственно. По объединенным данным двух исследований, снижение абсолютного числа нейтрофилов развивалось в среднем через 29–36,5 дней, медиана длительности нейтропении составила 11,5–15,0 дней.
Редукция дозы по поводу нейтропении потребовалась 10% пациенток в исследовании MONARCH-2 и 12,8% – в MONARCH-3. При этом процент пациенток, которым отменили терапию ввиду нейтропении, был низким и составил 1,6 и 2,8% соответственно. В остальных случаях с нейтропенией удалось справиться прерыванием терапии или редукцией дозы.
Важно отметить, что во всех исследованиях уровень нейтрофилов оставался стабильным после снижения и восстанавливался после окончания лечения, поскольку гематопоэтические клетки выходили из фазы ареста и приступали к делению.
Согласно текущей инструкции по медицинскому применению абемациклиба, для своевременного выявления нейтропении требуется регулярный мониторинг общеклинического анализа крови: контроль каждые 14 дней (день 1-й, 14-й) в течение 1-го и 2-го циклов, (день 1-й) циклов 3 и 4, далее по показаниям.
Препарат-специфическое НЯ: диарея, вызванная абемациклибом
Как уже было отмечено ранее, помимо высокоселективного ингибирования комплексов CDK4/CycD1 и CDK6/CycD1, абемациклиб связывается с CDK9. Согласно накопленным литературным данным, последняя ответственна за гастроинтестинальную токсичность, а именно, за развитие диареи.
По данным опубликованного сравнения токсичности при терапии различным iCDK4/6, диарея действительно оказалась более специфичной для абемациклиба: частота встречаемости данного НЯ в исследовании MONARCH-3 составила 65,8% (Gr.-3 – 6,76%) и 14,9% (Gr.-3 – 0%) в PALOMA-2 [36].
Развитие диареи не является предиктивным фактором ответа на абемациклиб: при оценке данных MONARCH-3 медиана ВБП у пациенток с диареей и без нее на фоне приема абемациклиба была сопоставимой: 28,2 и 29,1 месяца соответственно [37]. Характеристика диареи, развивавшейся на фоне абемациклиба (по данным исследований MONARCH), отражена в табл. 2.
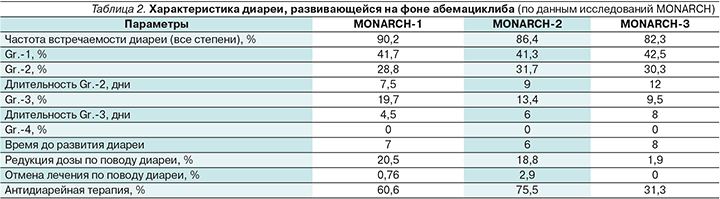
В монотерапии абемациклиб привел к развитию диареи всех степеней у 90,2% пациенток, в комбинации с фулвестрантом – у 86,4%, в комбинации с ИА – у 82,3% пациенток [38].
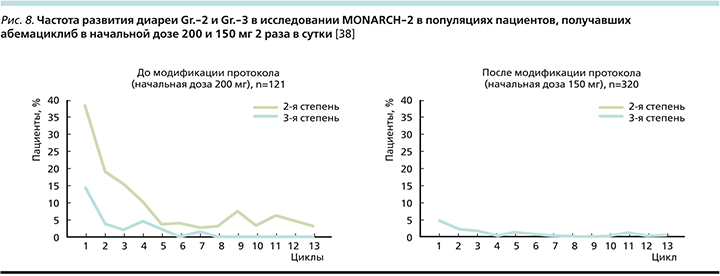
В исследовании MONARCH-2 первоначальная доза абемациклиба составляла 200 мг 2 раза в сутки, однако затем в условиях протокола была внесена корректировка дозы до 150 мг 2 раза в сутки в связи с высокой частотой развития именно диареи, приведшей к отмене терапии (6,6% до модификации и 1,6% после). До модификации протокола диарея Gr.-2 возникала у 43,8% пациентов, а после модификации – 27,2%; Gr.-3 – 19 и 11,3% до и после модификации соответственно (рис. 8).
Частота встречаемости диареи Gr.-1 во всех регистрационных исследованиях была сопоставимой и составила 41,3–42,5%. Частота развития диареи Gr.-2 в исследованиях MONARCH-1, -2 и -3 достигла 28,8%, 31,7 и 30,3% соответственно. Частота развития диареи Gr.-3 составила 19,7%, 13,4, 9,5%. Важно отметить, что ни у одной из пациенток не отмечено развития диареи Gr.-4.
Как правило, диарея на фоне терапии абемациклибом развивается на ранних сроках начала терапии: медиана времени до начала составляет приблизительно 7 дней, по данным исследований цикла MONARCH. При грамотной и своевременной коррекции режима терапии данное НЯ разрешается в достаточно сжатые сроки. Стоит отметить, что частота редукции дозы абемациклиба из-за диареи снижалась по мере эволюции исследований MON-ARCH (20,5–>18,8–>1,9%), поскольку появлялось больше понимания того, как правильно корректировать диарею. Как правило, диарея требовала назначения специализированных противодиарейных препаратов (в подавляющем большинстве случаев использовался лоперамид). С учетом высокой эффективности этого симптоматического препарата, доля пациенток, прервавших лечение из-за этого НЯ, составила всего лишь 0,76% в исследовании MONARCH-1, 2,9% – в MONARCH-2 и 0% – в исследовании MONARCH-3. Более высокий процент отмены терапии в MONARCH-2 объясняется вышеуказанной дозировкой абемациклиба – 200 мг 2 раза в сутки. Из 13 пациенток, прервавших терапию, 8 получали именно первоначально обозначенную дозировку.
Своевременная и правильная коррекция диареи крайне важна, поскольку данное НЯ напрямую влияет на качество жизни пациенток, а повышение качества жизни является одной из приоритетных целей лечения.
Согласно опубликованному анализу опросников European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 и Breast Cancer-Specific Quality of Life Questionnaire HRQo, которые оценивали качество жизни пациенток, получавших абемациклиб в исследовании MONARCH-3, оказалось, что статистически значимое снижение качества жизни пациенток наблюдалось только в отношении диареи [38].
В 2019 г. была опубликована статья Y. Omori et al., в которой рассматривались предпочтения пациенток в выборе лечения [39]. Пациенткам с HR+/HER2- мРМЖ предлагался выбор между двумя гипотетическими видами лечения в зависимости от показателей ВБП, частоты дефекации, частоты жидкого стула, а также путь приема препарата (пероральный или внутривенный). Оказалось, что 258 опрошенных пациенток готовы переносить диарею Gr.-2 для увеличения ВБП, однако при нарастании диареи до Gr.-3 пациентки были готовы пожертвовать увеличением ВБП и воздерживаться от лечения.
Данные этого опроса говорят о том, что крайне важно правильно корректировать это НЯ, чтобы не пришлось жертвовать высокой эффективностью.
Тактика ведения диареи, вызванной абемациклибом
Контроль данного НЯ может осуществляться двумя путями: редукцией дозы и приемом противодиарейных препаратов. В исследованиях MONARCH первым шагом для коррекции диареи был прием антидиарейных препаратов (лоперамид) при первых признаках жидкого стула. Если диарея не разрешалась в течение 24 часов от начала антидиарейной терапии, доза препарата редуцировалась на 50 мг, а полный отказ от приема абемациклиба рассматривался при непереносимости дозы 50 мг 2 раза в сутки.
В 2018 г. в Annals of Oncology были опубликованы рекомендации по ведению пациенток с диареей на фоне приема абемациклиба [40]. При развитии диареи Gr.-2, которая не купируется приемом антидиарейных средств, целесообразно приостановить прием абемациклиба до разрешения НЯ как минимум до Gr.-1, а при персистирующем течении (либо при повторном возникновении Gr.-2) – редуцировать дозу препарата. При развитии диареи Gr.-3 (или Gr.-4, что ни разу не встречалось в цикле исследований MONARCH) прием абемациклиба также приостанавливается до разрешения НЯ как минимум до Gr.-1, но при этом доза препарата должна быть редуцирована вне зависимости от срока разрешения.
Пациенткам необходимо предоставлять всю информацию об ожидающих НЯ лечения, готовить к появлению диареи и рекомендовать иметь при себе противодиарейный препарат (в подавляющем большинстве случаев – лоперамид).
Пациенткам также целесообразно корректировать образ жизни: придерживаться определенной диеты с исключением крестоцветных, избегать приема острой или пряной пищи, увеличивать в рационе продукты с высоким содержанием калия (бананы, картофель и т.п.), а также соблюдать питьевой режим во избежание дегидратации.
Профилактика диареи, вызванной абемациклибом
В появляющихся клинических исследованиях с участием абемациклиба подразумевается профилактика диареи. Так, в исследовании neoMONARCH предусматривался профилактический прием лоперамида по 2 мг при приеме каждой дозы абемациклиба [41]. Это ожидаемо позволило снизить частоту встречаемости диареи до 61%, при этом выраженность диареи первой степени (Gr.-1) составила 41%, более серьезной (Gr.-2) уже только 15%, Gr.3- – 5% и ни одного случая Gr.-4.
Новые достижения и перспективы применения абемациклиба
C учетом перспективности применения абемациклиба в мировой литературе встречается все больше и больше данных о применении препарата при различных клинических ситуациях и в различных комбинациях.
Так, например, исследование neoMONARCH показало эффективность абемациклиба в сочетании с ИА в неоадъювантном режиме лечения HR+/HER- РМЖ I–IIIb-стадий [41]. Помимо увеличения резектабельности опухоли отмечено достоверное снижение уровня Ki-67 после 2 недель терапии (пациенткам выполнялась повторная coreбиопсия).
Абемациклиб демонстрирует свое клиническое преимущество и в качестве адъювантной опции у радикально пролеченных пациенток с HR+/HER- РМЖ высокого риска прогрессирования [42, 43]. К такой группе относились пациентки, у которых отмечалось поражение либо 4 и более лимфатических узлов, либо 1–3 лимфатических узлов в сочетании с одним из следующих факторов: при размере первичной опухоли не менее 5 см, G3, уровне Ki-67 не менее 20%. Экспериментальная группа пациенток получила комбинацию классической эндокринотерапии в сочетании с абемациклибом. Выигрыш в ВБП инвазивного РМЖ на момент 2-летнего наблюдения составил 2,5%: 92,2% в экспериментальной группе и 88,7% в контрольной группе. Данные, конечно же, незрелые, но дают основание предполагать новую дополнительную опцию терапии для пациенток РМЖ высокого риска прогрессирования, имеющих крайне неблагоприятный прогноз при проведении им только стандартного адъювантного лечения.
Интересной опцией абемациклиб может стать и для пациенток с HR+/HER2+ мРМЖ [44]. В исследовании monarcHER сравнивалась эффективность комбинации абемациклиба и трустузумаба с фулвестрантом или без него (группы А и В соответственно) против комбинации трастузумаба и ХТ по выбору исследователя (группа С). При проведении попарного анализа оказалось, что группа А имела выигрыш в медиане ВБП в 2,6 месяца по сравнению с группой С (8,3 против 5,7 месяца). При этом разницы в ВБП между группами В и С отмечено не было. Ожидаемо, что токсичность лечения в группе А была значительно меньше, чем на фоне ХТ.
Заключение
Абемациклиб – уникальный представитель класса iCDK4/6. Во многом свойства препарата обусловлены его фармакокинетическими особенностями: например высокая селективность в ингибировании CDK4 при меньшей афинности к CDK6 приводит к более избирательной остановке клеточного цикла опухолевых клеток, большей проницаемости через гематоэнцефалический барьер и меньшей гематологической токсичности. В настоящее время проводится много клинических исследований, направленных на поиск оптимального места препарата в лечении разных подтипов РМЖ (как раннего, так и метастатического).


