Введение
Прошло более четверти века с момента публикации первого международного руководства по ведению взрослых больных внебольничной пневмонией (ВП) [1]. Этот документ, подготовленный экспертами Американского торакального общества и увидевший свет в 1993 г., не только оказался пионерским, но и имел далеко идущие последствия, радикально изменив существовавшие на тот момент подходы к диагностике и лечению пациентов данной категории.
В 1881 г. L. Paster [2] и G.M. Sternberg [3] независимо друг от друга описали микроорганизм, позднее названный A. Fraenkel пневмококком, что подчеркивало причинно-следственные связи между данным возбудителем и воспалением легких [4]. А два десятилетия спустя W. Osler не без доли сарказма определил клиническую сущность пневмонии как «надежного лоцмана человека на пути к его смерти» [5]. Введение в клиническую практику сывороточной терапии, особенно вакцин и антибиотиков, оказало значительное влияние на результативность лечения и профилактики пневмонии в течение ХХ в. Однако последние 50–60 лет не были отмечены никакими существенными нововведениями в терапии ВП. Одновременно с этим все чаще описываются новые возбудители заболевания (в частности, SARS-CoV, MERS-CoV, SARS-CoV-2) и патогены с множественной лекарственной устойчивостью, что вызывает обоснованное беспокойство во врачебном сообществе.
Все вышесказанное указывает на то, что пневмония остается серьезной проблемой общественного здравоохранения с неприемлемо высокой заболеваемостью и смертностью [6].
К числу вопросов, требующих решения, следует прежде всего отнести трудности в установлении первоначального клинического диагноза ВП, стратификацию риска неблагоприятного исхода заболевания, оптимизацию выбора эмпирической антибиотикотерапии, относительную нехватку новых антибиотиков и важность знания местных особенностей микробиологической чувствительности пневмотропных микроорганизмов. Между тем неопределенность в диагностике ВП также приводит к чрезмерному использованию антибиотиков и росту антибиотикорезистентности бактерий. К сожалению, с нетерпением ожидавшаяся новая версия согласительных рекомендаций Американского торакального общества/Американского общества инфекционных болезней [7] не смогла приблизиться к решению большинства из них.
Эпидемиология
Инфекции дыхательных путей (в т.ч. и ВП) остаются наиболее распространенными инфекционными заболеваниями в амбулаторной практике [8]. Однако определение истинной заболеваемости ВП остается сложной задачей, поскольку пациенты с легкими системными и респираторными симптомами нечасто обращаются за медицинской помощью. Так, например, на одного семейного врача во Франции в течение года приходится менее семь случаев ВП [9]. Здесь же следует принимать во внимание и то обстоятельство, что распространенность/доступность подтверждающих диагностических тестов (рентгенография органов грудной клетки и др.) на месте оказания медицинской помощи широко варьируется.
С учетом этих ограничений предполагаемая годовая заболеваемость ВП составляет 5–11 случаев на 1000 взрослых [10]. Важно также учитывать и выраженную сезонную вариабельность заболеваемости ВП (чаще заболевание наблюдается во второй половине осени–зимой), U-образное возрастное распределение (чаще у детей и пожилых людей), гендерную асимметрию (чаще у мужчин), а также ее заведомо бόльшую распространенность среди лиц с известными факторами риска (алкоголь, курение, хронические бронхолегочные заболевания, почечная недостаточность, дефицит питания и др.) или принимающих соответствующие лекарственные средства (ингаляционные глюкокортикостероиды, ингибиторы протонной помпы, антипсихотические препараты, ингибиторы дипептидилпептидазы-4) [11, 12].
Результаты популяционных исследований, проведенных в Европе, свидетельствуют, что частота ВП, требующей госпитализации, варьируется в диапазоне 1,98–2,6 на 1000 населения в год, что может отражать различия изучаемых групп населения и организации здравоохранения в той или иной стране [13, 14]. Отсюда следует, что около 75% больных получают лечение в амбулаторных условиях, поскольку заболевание в этих случаях протекает в нетяжелой форме и характеризуется достаточно низкой летальностью.
В противоположность летальность среди госпитализируемых больных достигает 5–15% [15, 16].
Лечение больных ВП обходится в США более чем в 8 млрд долл. ежегодно, причем более 90% этой суммы приходится на расходы на стационарное лечение [17]. Поэтому особое значение приобретает решение лечащего врача о госпитализации пациента и выписке его из стационара.
Диагностика
Проявления ВП включают респираторные (кашель, мокрота, одышка, боль в груди) и конституциональные симптомы (лихорадка, недомогание, гриппоподобные симптомы, нарушение сознания и др.), а также соответствующие физические проявления (тахипноэ, тахикардия, артериальная гипотензия, очаговая статоакустическая симптоматика – участок бронхиального дыхания, инспираторная крепитация, фокус мелкопузырчатых влажных хрипов, усиление бронхофонии). Поскольку эти проявления недостаточно чувствительны и специфичны для установления окончательного диагноза заболевания, рекомендуется проведение рентгенографии грудной клетки, подтверждающей в случае ВП наличие очагово-инфильтративных изменений в легких.
Следующие клинические данные повышают вероятность обнаружения инфильтративных изменений и должны побуждать к проведению рентгенологического исследования, в т.ч. и в амбулаторных условиях: а) отсутствие ринореи; б) одышка и/или учащенное дыхание; в) соответствующая очаговая аускультативная симптоматика; г) конституциональные симптомы – лихорадка, тахикардия >100/мин; д) повышенные концентрации биомаркеров (например, С-реактивный белок >20–30 мг/л). Если при этом подтверждается более двух из этих критериев, вероятность наличия инфильтрата легочной ткани у пациента с симптомами острой инфекции нижних дыхательных путей повышается с <5% до >18% [18].
Обнаружение очагово-инфильтративных изменений в легких на рентгенограмме обычно оказывается достаточным для того, чтобы надежно отличить ВП от острого бронхита. Последний, за редким исключением, не нуждается в лечении антибиотиками, поскольку побочные эффекты антибиотикотерапии в таких случаях перевешивают их благотворное влияние на симптомы [19]. Прокальцитонин является еще одним биомаркером, который может быть использован, чтобы помочь избежать ненужного назначения антибиотиков амбулаторным пациентам с инфекциями нижних дыхательных путей (14). Если точечные анализы недоступны, можно использовать подход «отсроченного назначения», минимизирующий необоснованную антибиотическую агрессию.
Стратификация
Затраты на госпитализацию, возможность возникновения внутрибольничных инфекций и тромбоэмболических осложнений требуют тщательного учета показаний к госпитализации больных ВП и выделение пациентов той категории, которые бы могли получать амбулаторное лечение без ущерба конечной эффективности и безопасности. Впечатление врача от клинической тяжести заболевания должно быть объективировано с помощью правильно обоснованных критериев. Определенные надежды в этом плане возлагались на шкалы оценки прогноза ВП, которые косвенно могли бы указывать на предпочтительное место лечения больного – на дому, в палатном отделении стационара или в отделении реанимации и интенсивной терапии (ОРИТ). И хотя эти шкалы оценки тяжести и прогноза ВП хорошо зарекомендовали себя при выявлении пациентов с высоким риском неблагоприятного исхода, они, строго говоря, не предназначались для выделения категории «амбулаторных больных» (табл. 1).
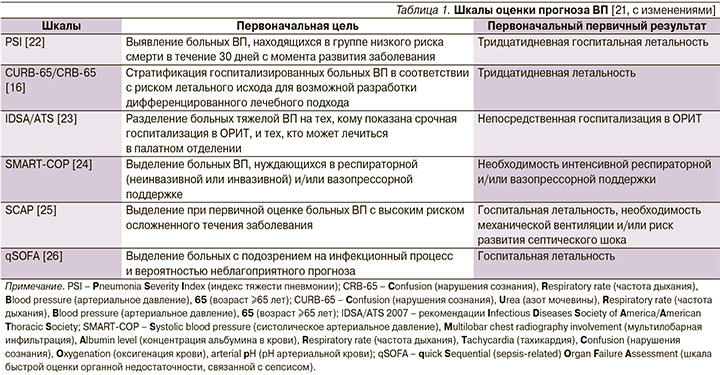
Из доступных в настоящее время шкал оценки прогноза ВП наиболее практичной в условиях первичной медицинской помощи оказалась шкала CRB-65, не требующая каких-либо лабораторных тестов и позволяющая достаточно безопасно выделять категорию больных (0 баллов) для амбулаторного лечения (табл. 2) [27, 28].
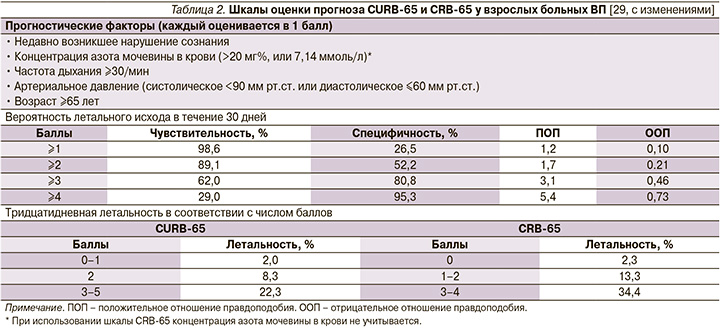
Тем не менее больные, перенесшие интеркуррентную пневмонию на фоне ряда заболеваний внутренних органов, несмотря на низкий балл по упомянутым шкалам могут иметь плохой прогноз [30–32]. В связи с этим отрицательная прогностическая ценность шкалы CRB-65 может быть значительно улучшена, если ее «обогатить» такими дополнительным параметрами, как показатель оксигенации гемоглобина кислородом (SaO2) по данным пульсоксиметрии, высокий риск обострения/декомпенсации сопутствующей патологии, факт длительного пребывания больного на постельном режиме (табл. 3).
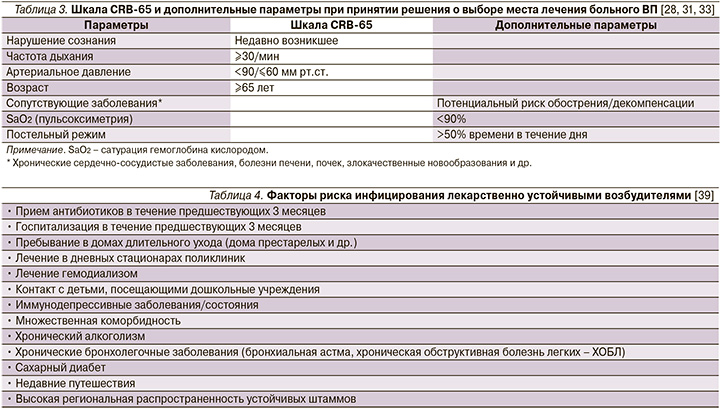
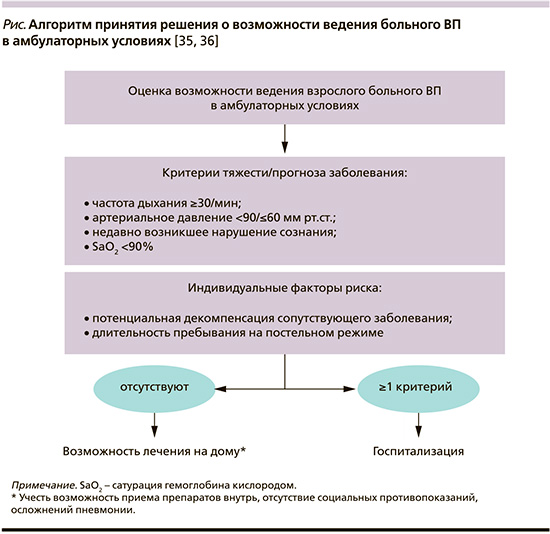
Подобная модификация шкалы CRB-65 присутствует, в частности, на страницах согласительных рекомендаций Немецкого респираторного общества, Немецкого химиотерапевтического общества им. Пауля-Эрлиха, Немецкого общества инфекционистов (German S3 guideline) при принятии решения о выборе места лечения больного ВП (см. рисунок) [34].
Антибактериальная терапия
Если врач считает целесообразным лечить больного ВП в амбулаторных условиях, антибактериальная терапия (АБТ) должна быть начата как можно раньше [7]. Более чем в половине случаев возбудителя/возбудителей ВП не удается идентифицировать, а с учетом значительных затрат на определение «виновного» микроорганизма становится очевидным, что пациенты в амбулаторных условиях лечатся эмпирически [37, 38]. В настоящее время выбор большинством экспертов антибиотика первой линии в лечении больных ВП без факторов риска «встречи» с лекарственно устойчивыми возбудителями (табл. 4) останавливается на амоксициллине (табл. 5).
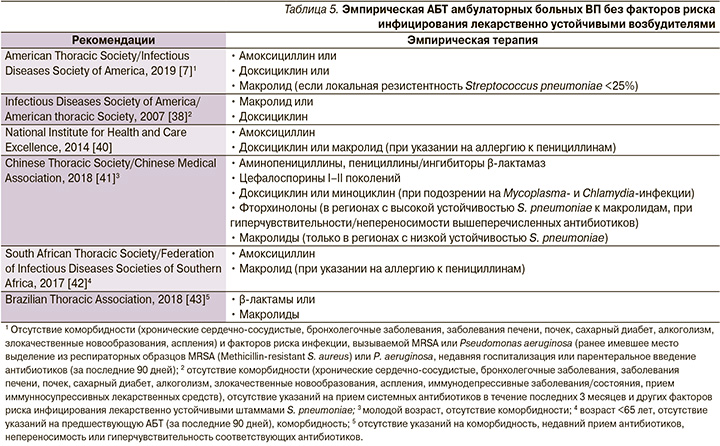
Этот выбор аргументируется результатами ряда исследований, свидетельствующих о высокой терапевтической эффективности амоксициллина в лечении ВП, несмотря на отсутствие активности в отношении «атипичных» возбудителей [7], доказанной безопасностью препарата по сравнению с другими вариантами антибиотикотерапии [44], драматическим ростом устойчивости пневмококка к макролидам в ряде стран и регионов, в т.ч. и в Российской Федерации [45]. Сказанное в отношении амоксициллина тем более важно, что в Кокрейновском обзоре, первоначально опубликованном в 2009 г., а затем дополненном и расширенном в 2014 г., при сравнении различных антибиотиков и групп антибиотиков в лечении взрослых амбулаторных больных ВП была продемонстрирована их сопоставимая эффективность [46, 47]. Впрочем, трудности в поиске доказательств превосходства того или иного режима эмпирической АБТ распространяются и на контингент больных ВП, требующих госпитализации. Так, в ходе недавно проведенного исследования при сравнении эффективности монотерапии β-лактамами, фторхинолонами, комбинации β-лактамов и макролидов не были установлены различия в 90-дневной кумулятивной летальности, а также медиане длительности стационарного этапа лечения [48].
У больных ВП, возникшей и протекающей на фоне хронических инвалидизирующих заболеваний внутренних органов (болезни сердца, легких, почек, печени, сахарный диабет, хронический алкоголизм, злокачественные новообразования и др.), рекомендуется применение антибиотиков более широкого спектра действия, что связано с повышенным риском инфицирования патогенами, устойчивыми к «незащищенным» пенициллинам (например, Haemophilus influenzae, Moraxella catarrhalis и Staphylococcus aureus), а также большей вероятностью неблагоприятных исходов в случаях неудачи стартовой терапии [7]. В обсуждаемых международными экспертами клинических рекомендациях предпочтение отдается «защищенным» аминопенициллинам (амоксициллин/клавуланат и др.) или цефалоспоринам II–III поколений, назначаемых в рамках монотерапии или в комбинации с макролидами или доксициклином (табл. 6).
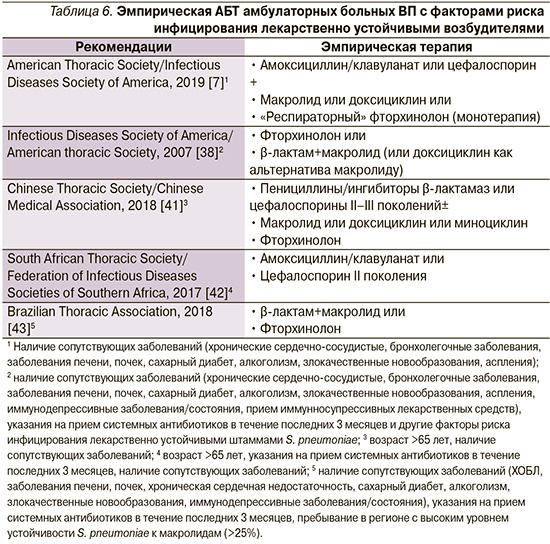
Очевидно, что имеющиеся различия (строго говоря, не носящие принципиального характера) в представленных подходах к эмпирической АБТ амбулаторных больных ВП в большей степени отражают национальные эпидемиологические данные антибиотикорезистентности ключевых возбудителей заболевания, их потенциальное экологическое влияние, а также учитывают затратную эффективность лекарственных средств. С этих позиций следует рассматривать и точку зрения Российского респираторного общества (РРО) и Межрегиональной ассоциации по клинической микробиологии и антимикробной химиотерапии (МАКМАХ). Так, на страницах проекта клинических рекомендаций, подготовленного экспертами РРО/МАКМАХ, предложено выделять две группы амбулаторных больных ВП [49]. В первую группу включены пациенты без хронических сопутствовавших заболеваний, не принимавшие за последние 3 месяца системных антибиотиков в течение 2 и более последовательных дней и не имевшие других факторов риска инфицирования редкими и/или полирезистентными возбудителями (ПРВ) – пребывание в доме престарелых или других учреждениях длительного ухода, наличие госпитализаций по любому поводу в течение ≥2 суток за последние 90 дней, внутривенная инфузионная терапия, наличие сеансов диализа или лечение ран в домашних условиях в предшествовавшие 30 дней. Во вторую группу включены больные ВП с сопутствовавшими заболеваниями (ХОБЛ, сахарный диабет, хроническая сердечная недостаточность, хроническая болезнь почек со снижением скорости клубочковой фильтрации, цирроз печени, алкоголизм, наркомания, дефицит питания) и/или принимавшие за последние 3 месяца антибиотики ≥2 дней и/или имевшие другие факторы риска инфицирования редкими и/или ПРВ, указанными выше.
Пациентам с ВП без значимых сопутствующих заболеваний и других факторов риска инфицирования редкими и/или ПРВ рекомендуется в качестве терапии выбора амоксициллин, альтернативы – макролиды (табл. 7).
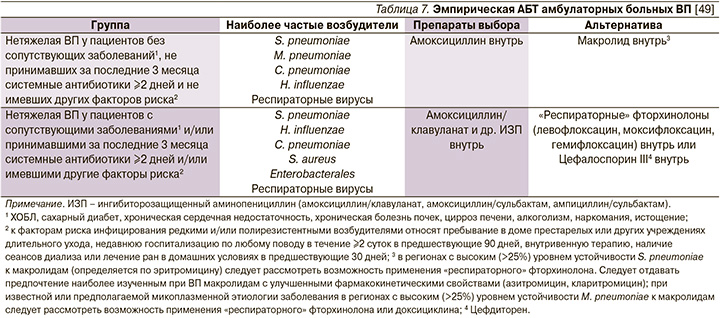
Позиция российских экспертов, отдающих предпочтение амоксициллину при лечении данной категории пациентов (и к слову, совпадающая с соответствующим положением большинства зарубежных рекомендаций), аргументируется рядом обстоятельств. Аминопенициллины сохраняет высокую активность в отношении ключевого возбудителя ВП в данной группе пациентов – S. pneumoniae, а частота выделения нечувствительных к амоксициллину изолятов H. influenzae в нашей стране остается невысокой [50, 51]. При этом, как уже упоминалось выше, хотя аминопенициллины in vitro и не перекрывают всего спектра потенциальных возбудителей ВП (в частности, не действуют на M. pneumoniae и Chlamydophila pneumoniae), в рандомизированных клинических исследованиях по эффективности они не уступают макролидам и респираторным хинолонам [52]. В связи со стремительным ростом устойчивости S. pneumoniae к макролидам в ряде регионов РФ и как следствие – увеличением риска терапевтической неудачи [53, 54] их назначение в качестве препаратов первого ряда не рекомендуется. Макролиды могут применяться при невозможности использовать аминопенициллины (индивидуальная непереносимость, аллергические реакции немедленного типа на бета-лактамы в анамнезе), а также при наличии клинических/эпидемиологических указаний на «атипичную» этиологию ВП [10, 42, 55, 56].
Больным ВП, у которых заболевание протекает на фоне значимых сопутствующих заболеваний и/или при наличии других факторов риска инфицирования редкими и/или ПРВ, рекомендуются в качестве антибиотиков выбора «защищенные» аминопенициллины (амоксициллин/клавуланат и др.), альтернативы – респираторные фторхинолоны или цефдиторен (см. табл. 7).
Подобный выбор больными второй группы объясняется большей вероятностью этиологической роли грамотрицательных бактерий (в т.ч. обладающих некоторыми механизмами вторичной антибиотикорезистентности) [42, 55, 57]. Альтернативой служат «респираторные» фторхинолоны (левофлоксацин, моксифлоксацин, гемифлоксацин) или цефдиторен. Фторхинолоны in vitro имеют определенные преимущества перед «защищенными» аминопенициллинами (более высокая активность в отношении энтеробактерий, действие на M. pneumoniae, C. pneumoniae, пенициллинорезистентные пневмококки), однако это не определяется при сравнении клинической эффективности. Кроме того, подобное «сдержанное» отношение к назначению фторхинолонов способно уменьшать селекцию лекарственно устойчивых возбудителей и сохранять возможность их использования при неэффективности антибиотиков первого ряда [58].
Несмотря на определенную роль «атипичных» возбудителей в этиологии ВП у пациентов данной группы, рутинное назначение комбинации β-лактамного антибиотика и макролида не рекомендуется, т.к. на сегодняшний день не доказано, что такая стратегия улучшает исходы лечения при возможном увеличении риска нежелательных лекарственных побочных эффектов и селекции антибиотикорезистентности [10, 40–42].
Эффективный клинический «ответ» больного ВП на АБТ обычно определяется стойкой апирексией, нормализацией частоты сердечных сокращений, дыхания, артериального давления и SaO2 (табл. 8) [23]. Для амбулаторных пациентов неудача лечения обычно определяется как необходимость госпитализации или коррекции АБТ спустя 48–72 часа.
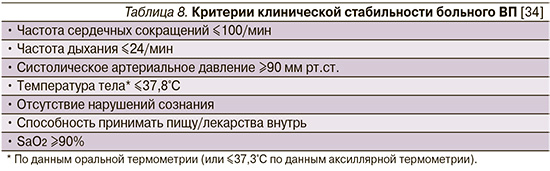
Хотя существует не так много данных, оценивающих отсутствие «ответа» на АБТ ВП в амбулаторных условиях, частота терапевтической неудачи, по-видимому, маловероятна и колеблется от 2,3 [59] до 7,5–8% [60, 61], что скорее всего связано с сопутствующими заболеваниями, а не с выбором антибиотиков или особенностями клинического течения самой пневмонии.
Длительность АБТ
Рекомендуемая продолжительность лечения для всех схем эмпирической АБТ больного ВП составляет не менее 5 дней, причем обычно 5–7 дней лечения считается достаточным для ведения пациента в амбулаторных условиях [7, 37, 62]. Подобные рекомендации строятся на результатах многочисленных рандомизированных контролируемых исследований и мета-анализов. Так, в частности, в ходе одного из них, включившего 21 клиническое исследование и 4861 амбулаторного и госпитализированного больного ВП, получавшего «короткую» (≤6 дней) и «длительную» (≥7 дней) терапию, были продемонстрированы сходные результаты клинического выздоровления между сравниваемыми группами (отношение риска (ОР)=0,99; 95% доверительный интервал (ДИ)=0,97–1,01) [63]. При этом результативность «короткий» и «длительной» АБТ оказалась сопоставимой в группе как амбулаторных (ОР=0,98, 95% ДИ: 0,97–1,01), так и госпитализированных больных (ОР=1,00, 95% ДИ: 0,92–1,09). Небезынтересным оказался и тот факт, что «короткие» курсы ассоциировались с меньшим числом серьезных побочных эффектов (ОР=0,73, 95% ДИ: 0,55–0,97) и более низкой кумулятивной летальностью (ОР=0,73, 95% ДИ: 0,55–0,97) по сравнению с «традиционной» или «длительной» продолжительностью лечения.
Впрочем, если подходить к вопросу об оптимальной продолжительности АБТ взрослых больных ВП в амбулаторных условиях более строго, то следует признать, что до настоящего времени не проведено ни одного рандомизированного контролируемого исследования, в рамках которого изучались бы эффективность и безопасность короткого и длительного («традиционного») курсов лечения (с одним и тем же антибиотиком и в той же суточной дозировке). А значит, влияние продолжительности АБТ на течение и исходы ВП для взрослых амбулаторных пациентов остается неясным [64].
Новые антибиотики
Наряду с популяризацией подходов к рациональной АБТ ВП, в т.ч. и избеганием необоснованной антибиотической «агрессии» при небактериальных инфекциях дыхательных путей (по самым скромным оценкам, доля больных заведомо вирусными респираторными инфекциями, получающими антибиотики, превышает 50%) [65, 66], еще одной действенной стратегией в противоборстве с глобальной пандемией антибиотикорезистености является создание и внедрение в повседневную клиническую практику новых антибиотиков. Из их числа наиболее перспективными для амбулаторного лечения взрослых больных ВП представляются омадациклин и лефамулин (табл. 9). Омадациклин – это новый антибиотик широкого спектра действия, относящийся к классу тетрациклинов, назначаемый внутрь или внутривенно 1 раз в сутки и одобренный FDA (Food and Drug Administration) в октябре 2018 г. в качестве лечения ВП [67].
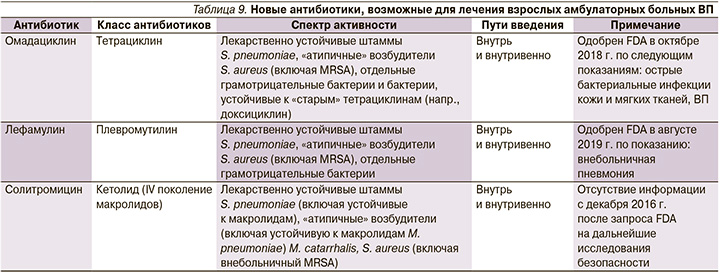
В августе 2019 г. FDA для лечения больных ВП одобрило лефамулин, относящийся к подклассу плевромутилинов [68], антимикробное действие которых объясняется ингибированием синтеза бактериальных белков [69]. Новый антибиотик доступен в лекарственных формах для перорального и внутривенного введений. Недавно были опубликованы результаты двух исследований, продемонстрировавших сопоставимую клиническую эффективность (non-inferiority) лефамулина и моксифлоксацина [70, 71]. При этом T.M. File et al. [70] свидетельствовали о сходных частоте и структуре нежелательных лекарственных реакций (НЛР) лефамулина и моксифлоксацина, тогда как в публикации, описывающей исследование E. Alexander et al. [71], сообщалось о большей частоте нетяжелых НЛР в группе пациентов, принимавших новый антибиотик. Последнее обстоятельство, а также стоимость лефамулина (цена суточной дозы препарата составляет 275 USD, что существенно дороже стоимости фторхинолона), тем не менее не мешают видеть в этом антибиотике новое перспективное направление лечения ВП, в т.ч. и в амбулаторных условиях [72, 73].
Профилактика
Гриппозная и пневмококковая вакцины общепринято рекомендуются в качестве профилактической меры лицам старше 60 лет и больным, страдающим рядом неинфекционных инвалидизирующих заболеваний внутренних органов. Данные о профилактической эффективности гриппозной вакцины для больных ВП ограниченны, тем не менее ее применение признается оправданным ввиду доказанной эффективности против гриппа, а также значимости гриппозной инфекции в развитии ВП и тяжести ее течения [74, 75].
С целью специфической профилактики пневмококковых инфекций, в т.ч. пневмококковой ВП у взрослых, используются вакцины двух типов: 23-валентная пневмококковая полисахаридная вакцина (ППСВ23) и 13-валентная пневмококковая конъюгированная вакцина (ПКВ13). [76–79]. Согласно проекту рекомендаций РРО/МАКМАХ [49], всем пациентам с высоким риском развития пневмококковых инфекций1 рекомендуется иммунизация пневмококковыми вакцинами. Лиц в возрасте 65 лет и старше, а также иммунокомпрометированных пациентов2 рекомендуется первоначально вакцинировать однократно ПКВ13, а затем (не позднее, чем через 12 месяцев) – ППСВ23 с последующей ревакцинацией ППСВ23 каждые 5 лет. Пациентам 18–64 лет, относящимся к группе высокого риска развития пневмококковой инфекции, но не являющимся иммунокомпрометированными, рекомендуется вакцинация ППСВ23 однократно. Сходной считается и точка зрения Комитета советников по иммунизационной практике [82].
Поскольку курение – ведущий фактор риска развития хронического бронхита/ХОБЛ, очевидна необходимость более широкого распространения программ по борьбе с курением как эффективного способа уменьшить риск развития ВП [83]. К числу других неспецифических, но достаточно действенных профилактических подходов следует относить оптимизацию лечения сопутствующих сердечно-сосудистых и бронхолегочных заболеваний, выявление и лечение возможной дисфагии с целью предотвращения аспирации. Cтрогая переоценка показаний к применению тех групп лекарственных средств, которые могут обусловить развитие ВП (седативные и антипсихотические препараты, ингибиторы протонной помпы, ингаляционные глюкокортикостероиды при ХОБЛ), плюс оптимальная гигиена полости рта [34] являются еще одними из важных мер профилактики пневмонии, серьезность которых зачастую недооценивается в рутинной клинической практике.
Заключение
Несмотря на то что, согласно существующей практике в странах Западной Европы и Северной Америки, абсолютное большинство больных ВП получают лечение в амбулаторных условиях, до настоящего времени основная масса исследований, направленных на поиск оптимальных терапевтических подходов при этом заболевании, касается госпитализированных пациентов.
С момента публикации первых клинических рекомендаций по диагностике, лечению и профилактике ВП у взрослых в 1993 г. [1] основные проблемы по ведению больных ВП, а именно: установление первоначального диагноза, стратификация пациентов по степени риска неблагоприятного исхода заболевания, эмпирический выбор антибиотиков, понимание важности локальных моделей чувствительности респираторных патогенов и др., сохраняют свою актуальность. Активное развитие в последние годы молекулярной биологии и популяризация методов генного зондирования существенно видоизменили и расширили наши традиционные представления об этиологии ВП (продемонстрировав, в частности, значительный «удельный вес» вирусов в ряду причин заболевания). Еще одна проблема – повсеместный рост устойчивости возбудителей к антибиотикам, что актуализирует важность создания новых антибиотиков и популяризацию действенных направлений и методов профилактики ВП.
____________________
1 К группам высокого риска развития пневмококковых инфекций относятся [80, 81]:
• пациенты в возрасте 65 лет и старше;
• лица с сопутствующими хроническими заболеваниями бронхолегочной (ХОБЛ, бронхиальная астма в сочетании с хроническим бронхитом и эмфиземой, принимающих длительно системные глюкокортикостероиды), сердечно-сосудистой систем (ишемическая болезнь сердца, хроническая сердечная недостаточность, кардиомиопатии и др.), сахарным диабетом, хроническими заболеваниями печени (включая цирроз), хронической болезнью почек, нефротическим синдромом, алкоголизмом, кохлеарными имплантами, ликвореей, функциональной или органической аспленией (серповидно-клеточная анемия, спленэктомия);
• пациенты с иммунодефицитом (ВИЧ-инфекция, злокачественные новообразования, иммуносупрессивная терапия и др.);
• лица, проживающие в домах престарелых и других учреждениях закрытого типа;
• курильщики.
2 К иммунокомпрометированным относятся пациенты с врожденными и приобретенными иммунодефицитами (в т.ч. ВИЧ-инфекцией и ятрогенными иммунодефицитами); пациенты, страдающие нефротическим синдромом, хронической болезнью почек и требующие диализа; лица с кохлеарными имплантами (или подлежащие кохлеарной имплантации); ликвореей; пациенты, страдающие гемобластозами и получающие иммуносупрессивную терапию; лица с врожденной или приобретенной (анатомической или функциональной) аспленией; гемоглобинопатиями (в т.ч. серповидно-клеточной анемией); находящиеся в листе ожидания на трансплантацию органов или после таковой [81].


