Введение
Получение новых данных о гетерогенности люминального метастатического рака молочной железы (ЭР+ HER2- мРМЖ) способствовало поиску терапевтических агентов, биологически направленных на различные жизненно важные сигнальные пути, приводящих в конечном итоге к необратимым изменениям клеточного цикла опухолевой клетки. Все большее внимание уделяется необходимости одновременного воздействия на несколько мишеней, что позволяет преодолевать резистентность, возникающую в результате перекрестного патогенетического взаимодействия между сигнальными путями, в т.ч. активации рецепторов тирозинкиназы и т. д. [1–4]. Изучается несколько важных вопросов, касающихся последовательности лечения, комбинаторных стратегий, что способствует дальнейшему расширению и совершенствованию клинического применения ингибиторов циклинзависимых киназ-4 и -6 (CDK4/6) в лечении больных гормонопозитивным РМЖ.
За последние 4 года FDA одобрено 3 низкомолекулярных ингибитора CDK4/6: рибоциклиб, палбоциклиб и абемациклиб, для комбинированной стратегии эндокринной терапии в качестве предпочтительного метода для лечения больных ЭР+ HER2-мРМЖ в отсутствие висцерального криза. Применение различных пероральных ингибиторов CDK4/6 уже сегодня позволяет значительно улучшить показатели выживаемости больных ЭР+ HER2- мРМЖ. Профиль токсичности препаратов данного класса хорошо известен и предсказуем [5–14].
Роль CDK4/6 в жизнедеятельности опухолевой клетки
Представления о причастности циклинзависимых киназ к процессам злокачественной трансформации подтверждаются экспериментами на лабораторных животных. При помощи генноинженерных процессов у ряда мышей был «повержен» ген CDK4, что привело к снижению их чувствительности к канцерогенам. Абемациклиб по своей структуре отличается от других ингибиторов CDK4/6 (таких, как рибоциклиб и палбоциклиб). По данным ферментных анализов абемациклиб проявляет активность в отношении комплекса циклин D1/CDK4, в 14 раз более высокую, чем в отношении комплекса циклин D3/CDK6 [15–17]. Как показали данные доклинических исследований, для пролиферации, индуцированной эстрогеновыми рецепторами (ЭР), требуется циклин D [18, 19], который экспрессирован в высокой степени клетками РМЖ более чем у 50% пациенток [20].
Регулирование клеточного цикла осуществляется десятками молекул, нарушение деятельности которых запускает процесс злокачественной трансформации [21]. Комплекс циклин D1-CDK4/6-RB1 является основным медиатором клеточной пролиферации, опосредованной передачей сигналов эстрогена [22–24]. Эстроген индуцирует экспрессию циклина D1 [25–27] и тем самым способствует активности CDK4/6 в клетках ЭР+ HER2- мРМЖ, приводя к гиперфосфорилированию RB1 и продвижению клеточного цикла [28]. CDK4/6 в комплексе с циклинами D-типа фосфорилируют белок ретинобластомы (Rb), что приводит к высвобождению фактора E2F, связанного с белком Rb. Фактор E2F в свою очередь способствует прохождению клеточного цикла из G1-фазы в S-фазу [29, 30]. Прямое подавление CDK 4/6 приводит к разрыву этого пути и подавлению роста клеток РМЖ. Данные доклинических исследований показали, что краткосрочное ингибирование CDK4/6 вызывает временную остановку клеточного цикла в фазе G1, который возобновляется после отмены подавления [16]. Однако непрерывное длительное ингибирование CDK4/6 приводит к устойчивой остановке роста, инициации апоптоза и старению клеток [31].
Преклинические и клинические исследования CDK4/6 на дорегистрационном этапе
Абемациклиб является избирательным низкомолекулярным ингибитором CDK 4/6 для непрерывного приема внутрь 2 раза в сутки [15, 16, 32]. Доклинические данные показали новую потенциальную роль абемациклиба как стимулятора противоопухолевого иммунитета за счет повышения презентации антигенов и избирательного ингибирования пролиферации регуляторных Т-клеток. Это указывает на возможность участия в лечебном эффекте различных механизмов действия абемациклиба, приводящих к уменьшению размера опухоли [33]. Исследование IВ-фазы, включившее 47 пациенток с частотой клинического ответа 61,1%, дало начало разработке клинического исследования MONARCH-1 по оценке эффективности монотерапии абемациклибом больных, интенсивно предлеченных по поводу ЭР+ HER2- мРМЖ [34].
Воздействие абемациклиба на клетки РМЖ, экспрессирующие ЭР, приводило к снижению фосфорилирования Rb, остановке клеточного цикла в фазе G1 и уменьшению пролиферации клеток. Также отмечалась реакция старения, о чем свидетельствуют накопление β-галактозидазы, образование связанных со старением очагов гетерохроматина и уменьшение количества FOXM1-позитивных клеток.
В ксенотрансплантатной модели ЭР+ РМЖ монотерапия абемациклибом вызывала регресс опухолевого роста. Противоопухолевую активность абемациклиба in vivo оценивали у мышей, которым имплантировали ксенотрансплантат ZR-75-1 (модель люминального ЭР+ рака молочной железы человека). Дозозависимая противоопухолевая активность наблюдалась после применения абемациклиба в качестве единственного агента в дозе 75 мг/кг [17].
Исследования с применением ксенотрансплантатов на грызунах, проведенные другой группой исследователей, показали, что абемациклиб может преодолевать гематоэнцефалический барьер [35].
В исследовании I фазы с эскалацией дозы абемациклиба у больных мРМЖ (включая одну когорту пациенток с ГР+ мРМЖ, получавших абемациклиб в комбинации с фулвестрантом) был продемонстрирован фармакокинетический профиль препарата, свидетельствующий об отсутствии необходимости корректировки дозы в зависимости от массы тела пациенток, возраста или пола [36]. В исследование I фазы включали интенсивно предлеченных больных с висцеральными метастазами, получавших в среднем 7 линий предшествовавшей терапии. Активность абемациклиба была продемонстрирована в режиме монотерапии с частотой ответа 23% и медианой выживаемости без прогрессирования (ВБП) 5,8 месяца [15]. В данное исследование не закладывалась оценка противоопухолевого ответа в зависимости от гормонального статуса. Однако был отмечен более высокий уровень контроля заболевания у 81% пациенток с ГР+ РМЖ против 70% в общей когорте и против 33% в подгруппе с ГР- РМЖ. Аналогичные результаты в представленных группах также были отмечены в отношении медианы продолжительности ответа и медианы ВБП. Это исследование включило отдельную когорту пациенток с эскалацией дозы 1 раз в день и 2 раза в день с использованием схемы «3+3». Дозолимитирующая токсичность не наблюдалась у пациенток, получавших абемациклиб 1 раз в день в предписанной максимальной дозе 225 мг/день, поэтому максимальная переносимая доза не была достигнута. В группе пациенток, получавших абемациклиб 200 мг каждые 12 часов, у одной из 7 больных отмечена дозолимитирующая токсичность в виде усталости 3-й степени. У когорты пациенток, получавших абемациклиб в дозе 275 мг каждые 12 часов, об усталости 3-й степени сообщили двое из трех больных. Таким образом, рекомендованная исследовательская доза II фазы была установлена на уровне 200 мг каждые 12 часов [15].
Регистрационные клинические исследования Абемациклиба
MONARCH-2 – рандомизированное двойное слепое плацебо-контролируемое исследование III фазы по оценке фулвестранта в комбинации с абемациклибом и без такового у женщин с ГР+ HER2- мРМЖ и прогрессированием заболевания на фоне предшествовавшей эндокринной терапии
Впервые результаты исследования MONARCH-2 были опубликованы в 2017 г. В 2019 г. были представлены результаты в отношении общей выживаемости (ОВ). В исследование включали женщин в возрасте ≥18 лет с любым менопаузальным статусом (женщины в пре- и перименопаузе также получали агонист гонадотропин-рилизинг гормона) и оценкой общего состояния по критериям ECOG 0–1.
Основной первичной оценочной точкой был принят критерий ВБП, оцениваемый от даты рандомизации до объективного прогрессирования заболевания или смерти больного по любой причине. Второстепенные критерии включали частоту объективного ответа (ОО), длительность ремиссии и переносимость.
Медиана ВБП в группе абемациклиба в комбинации с фулвестрантом достигла 16,4 месяца против 9,3 в контрольной группе (относительный риск [ОР]=0,553; 95% доверительный интервал [ДИ]: 0,449–0,681; р<0,001. Слепой централизованный анализ показал сходные результаты (ОР=0,460; 95% ДИ: 0,363–0,584; p<0,001). Частота ОО составила 35,2% (95% ДИ: 30,8–39,6) в группе абемациклиба и 16,1% (95% ДИ: 11,3–21,0) в контрольной группе (p<0,001). В этот показатель были включены 14 случаев полного регресса (ПР; 3,1%) в группе абемациклиба и 1 случай ПР (0,4%) в контрольной группе (табл. 1). Ремиссия в обеих группах была продолжительной. Ремиссия длительностью 12 месяцев зарегистрирована у 67,8% пациентов в группе абемациклиба и 66,9% – в группе плацебо [5, 7].
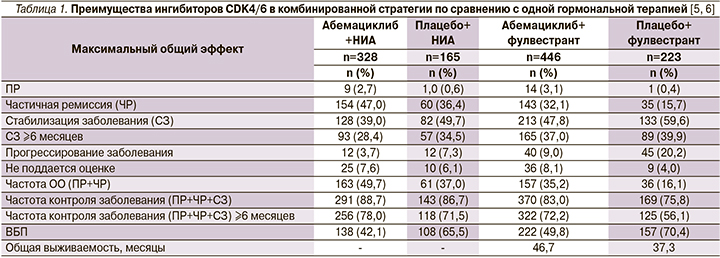
Профиль токсичности препарата представлен в табл. 2. Диарея 1-й и 2-й степеней наблюдалась у 73,0% (322) пациенток в группе абемациклиба против 24,2% (54) – в группе плацебо. Диарея 3-й степени регистрировалась реже: 13,4% (n=59) в группе абемациклиба против 0,4% (n=1) в группе плацебо. В группе абемациклиба диарея обычно начиналась на первом цикле лечения (медиана срока наступления диареи составила 6 дней). В большинстве случаев диарею удавалось эффективно купировать применением противодиарейных лекарственных средств и модификацией дозы абемациклиба.
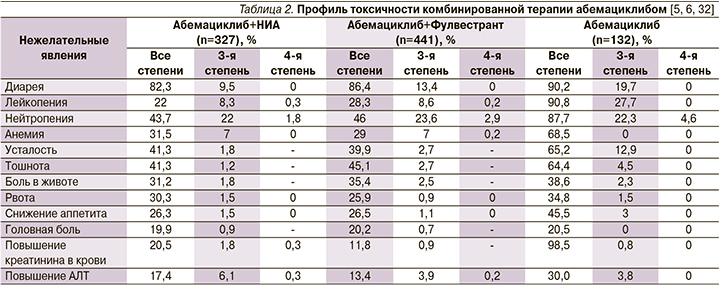
В группе абемациклиба у 14,5% пациенток с диареей 2-й степени и у 1,1% пациенток с диареей 3-й степени на начальном этапе отмечалось повторное появление диареи той же или более тяжелой степени тяжести. В большинстве случаев (70,1%) пациентам с диареей не требовалось изменения режима назначения абемациклиба (перерыв в дозировании, редукция дозы или отмена лечения) в связи с нежелательными явлениями. В группе абемациклиба частота возрастания уровня креатинина в сыворотке была на 25% выше, чем в группе плацебо. Показано, что применение абемациклиба приводило к повышению уровня креатинина в сыворотке в результате подавления его секреции почечными канальцами без ухудшения функции клубочков. Другие негематологические нежелательные явления (НЯ), часто отмечавшиеся в группе абемациклиба, включали тошноту, усталость и боль в животе (табл. 2).
На фоне лечения или в первые 30 дней после окончания терапии в группе абемациклиба в комбинации с фулвестрантом умерли 3,2% (14) пациенток (9 по причине НЯ) против 4,5% (10) в контрольной группе (2 по причине НЯ). НЯ, классифицируемые как связанные с исследуемым препаратом, отмечены в 0,7% случаев (3 летальных исхода): 2 – в результате сепсиса у больных с несоблюдением рекомендаций относительно применения гранулоцитарного колониестимулирующего фактора и редукции дозы и 1 – в результате вирусной пневмонии у пациентки, получавшей глюкокортикостероиды по поводу компрессии спинного мозга [5, 7].
На основании результатов регистрационного исследовании MONARCH-2 в сентябре 2017 г. абемациклиб был зарегистрирован FDA в комбинации с фулвестрантом для лечения женщин с ЭР+ HER2- местнораспространенным/метастатическим РМЖ и прогрессированием заболевания после эндокринной терапии [5, 38].
MONARCH-3: рандомизированное плацебо-контролируемое исследование III фазы по изучению комбинации абемациклиба с нестероидными ингибиторами ароматазы (НИА) летрозолом или анастрозолом и монотерапии НИА (+плацебо) в 1-й линии лечения ЭР+ HER2- мРМЖ
В исследовании III фазы MONARCH-3 были рандомизированы 493 пациента для получения абемациклиба в комбинации с НИА (n=328) или плацебо в комбинации с нестероидными ИА (n=165; табл. 1). Предшествовавшую неоадъювантную или адъювантную эндокринную терапию получали 46,7% (230) пациенток, предшествовавшую неоадъювантную или адъювантную химиотерапию – 38,7% (191). На момент окончания регистрации данных для окончательного анализа ВБП было зарегистрировано 246 событий: 138 (42,1%) в группе абемациклиба и 108 (65,5%) в группе плацебо. Медиана периода последующего наблюдения составила 26,73 месяца. Медиана ВБП, по оценке исследователя, составила 28,18 месяца в группе абемациклиба и 14,76 в группе плацебо (ОР=0,540; 95% ДИ: 0,418–0,698; р=0,000002). Эти оценки ВБП согласовывались с независимой централизованной оценкой этого показателя (ОР=0,465; 95% ДИ: 0,339–0,636; p<0,000001). Анализ ВБП в подгруппах пациентов показал, что добавление абемациклиба к НИА было эффективным во всех подгруппах.
У пациенток, получавших абемациклиб, эффект обычно отмечался в первые восемь циклов лечения. Медиана длительности ремиссии была значительно больше в группе абемациклиба (27,39 месяца), чем в группе плацебо (17,46 месяца) [39].
Анализ периода отсутствия лечения (ПОЛ), проведенный в рамках исследования MONARCH-3, с распределением больных в две группы в зависимости от величины ПОЛ (<36 или ≥36 месяцев) показал, что у пациенток с более длительным ПОЛ отмечалась более продолжительная ВБП. Анализ в подгруппах, основанный на данных ПОЛ, показал, что у пациенток с ПОЛ <36 месяцев добавление абемациклиба к нестероидному НИА улучшало результаты лечения, а также увеличивало частоту ОО на 32,11% [37].
Профиль токсичности изучаемого режима подробно представлен в табл. 2.
В группе абемациклиба более продолжительное последующее наблюдение подтвердило, что диарея 1-й и 2-й степеней наблюдалась в 72,8% случаев, начиналась на первом цикле у 69,1% пациенток и успешно купировалась в большинстве случаев. Нейтропения отмечалась у 43,7% пациенток, причем у 23,9% – 3–4-й степеней. Если по данным промежуточного анализа у одного пациента отмечалась фебрильная нейтропения, то ко времени окончательного анализа новых случаев этого НЯ не отмечено (табл. 3).
В феврале 2018 г. абемациклиб был зарегистрирован FDA для применения в комбинации с НИА на основании данных исследования MONARCH-3 (NCT02246621). По данным промежуточного анализа исследования MONARCH-3 по применению абемациклиба в комбинации с НИА было получено статистически значимое увеличение ВБП и повышение частоты ОО у больных в первой линии терапии ЭР+ HER2- мРМЖ [6].
С целью определения статистически значимых прогностических факторов был проведен комбинированный анализ исследований MONARCH-2 и MONARCH-3. Анализ клинических факторов подтвердил прогностическую ценность следующих из них: метастатическое поражение только костей, метастазы в печени, степень злокачественности опухоли, статус рецепторов прогестерона, соматический статус, ПОЛ с момента окончания адъювантной эндокринотерапии и время от момента постановки диагноза до рецидива. Среди пациенток с неблагоприятными прогностическими факторами эффект добавления к терапии абемациклиба был максимальным. К таким факторам неблагоприятного прогноза относили метастазы в печени, отрицательный статус рецепторов прогестерона, агрессивное течение (ПОЛ <36 месяцев с момента окончания адъювантной эндокринотерапии и короткий период с момента постановки диагноза) [37].
MONARCH-1: исследование II фазы по оценке ингибитора CDK4/6 абемациклиба в режиме монотерапии пациентов с рефрактерным ЭР+ HER2- мРМЖ
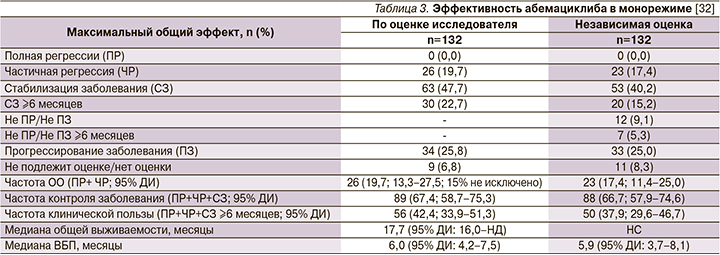
В исследование были включены 132 пациентки с ЭР+ HER2-мРМЖ. У всех пациенток на момент включения в исследование определялись измеряемые опухоли в соответствии с требованием протокола (RECIST v. 1.1).
У большинства больных (90,2%) зарегистрированы метастазы во внутренние органы и у 50,8% ≥3 органов метастазирования; чаще всего органами метастазирования являлись печень и кости. Абемациклиб в режиме монотерапии показал противоопухолевую активность интенсивно предлеченных пациентов с ЭР+ HER2- мРМЖ. Частота ОО составила 19,7% (95% ДИ: 13,3–27,5); частота клинически значимого ответа – 42,4%. Подтвержденная ЧР отмечена у 26 из 132 пациентов, что соответствовало 19,7% (95% ДИ: 13,3–27,5); случаев ПР зарегистрировано не было (табл. 3).
Из 26 больных ЭР+ HER2- мРМЖ с ЧР 12 (46,2%) получали не менее двух режимов предшествовавшей химиотерапии по поводу метастатического заболевания, у 24 (92,3%) определялись метастазы во внутренние органы и у 12 (46,2%) было ≥3 органов метастазирования [32].
Медиана ВБП достигла 6,0 месяцев. Медиана длительности ремиссии составила 8,6 месяца, медиана времени до наступления эффекта – 3,7 месяца.
По данным исследования MONARCH-1, диарея отмечена у 90,2% (119) пациенток: 1-й степени – у 41,7% (55); 2-й – у 28,8% (38); реже наблюдалась диарея 3-й степени – 19,7% (26) пациенток. Медиана времени до начала диареи составила 7,0 дней. Важно отметить, что продолжительность диареи не была значительной: медиана продолжительности диареи 2-й степени составила 7,5 дней, 3-й степени – 4,5; диарея 4-й степени не зарегистрирована. Отмена лечения в связи с диареей потребовалась всего 1 (0,8%) пациентке. Повышение уровня креатинина в сыворотке чаще всего наблюдалось в течение первого цикла; далее он оставался повышенным, но стабильным в течение всего периода терапии и снижался через небольшое время в период последующего наблюдения (в первые 30 дней после окончания лечения по протоколу исследования). Снижение уровня нейтрофилов наблюдалось у 114 (87,7%) пациенток: нейтропения 1-й или 2-й степени – у большинства больных, нейтропения 3-й степени – у 22,3% и нейтропения 4-й степени – у 4,6% пациенток. Нейтропения обычно достигала минимума между 2-й и 4-й неделями от начала лечения и оставалась стабильной в течение всего периода терапии абемациклибом (табл. 2).
Таким образом, непрерывное применение абемациклиба в режиме монотерапии больных ЭР+ HER2- мРМЖ с неблагоприятным прогнозом, получавших интенсивное предшествовавшее лечение, показало не только обнадеживающую эффективность, но и удовлетворительную переносимость.
Абемациклиб – единственный ингибитор CDK4/6 был одобрен FDA в сентябре 2017 г. для применения в режиме монотерапии больных ЭР+ HER2- мРМЖ, получавших интенсивное предшествовавшее лечение, на основании регистрационного исследования MONARCH-1 [32, 38].
Алгоритм профилактики и лечения осложнений терапии абемациклибом
Необходимо проводить полный анализ крови до начала терапии абемациклибом и каждые 2 недели в течение первых 2 месяцев лечения, а затем ежемесячно в течение следующих 2 месяцев терапии. Приостановка приема абемациклиба или снижение дозы рекомендуется пациентам, у которых развивается нейтропения 3-й или 4-й степени тяжести.
При первых признаках диареи рекомендуется начать использовать лоперамид и увеличить потребление жидкости. В тяжелых случаях применение абемациклиба необходимо приостановить, затем возобновить в более низкой дозе. В клиническом исследовании MONARCH-1 многие пациентки (60,6%) получали препараты против диареи, в 94% случаев это был лоперамид [32].
Редукция дозы абемациклиба
По данным исследования MONARCH-1, редукция дозы в связи с НЯ потребовалась 65 (49,2%) пациенткам (46 больным – 1 редукция, 18 пациенткам – 2 и 1 пациентке – 3).
В большинстве случаев редукция дозы была обусловлена развитием диареи (n=27; 20,5%) или нейтропении (n=14; 10,6%). Пропуск дозы требовался 76 (57,6%) пациенткам, чаще всего по причине диареи (n=32; 24,2%) и нейтропении (n=21; 15,9). Отмена лечения в связи с НЯ потребовалась в небольшом числе случаев (n=10; 7,6%). Обычно период пропуска дозирования был непродолжительным. Медиана числа пропущенных доз составила 6,5% от назначенного режима, что соответствовало медиане относительной дозовой интенсивности 89,2%. Большинство пациенток, участвовавших в исследовании MONARCH-1, смогли продолжить лечение с использованием химиотерапии [32].
Исследование MONARCH-2 показало, что в группе пациенток, получавших абемациклиб в комбинации с фулвестрантом, отмена абемациклиба по причине НЯ потребовалась в 15,9% (70) наблюдения, отмена плацебо в связи с НЯ – 3,1% (7), причем у 2,9% пациенток исследуемое лечение было отменено из-за диареи. Редукция дозы в связи с НЯ проведена 42,9% (189) пациенткам в группе абемациклиба и 1,3% (3) больным, получавшим плацебо. Перерыв в применении абемациклиба требовался в 51,9% (229) случаев, в применении плацебо – в 11,7% (26) [7].
По данным анализа результатов исследования MONARCH-3, при увеличении времени последующего наблюдения и непрерывном применении абемациклиба частота модификации дозы в группе абемациклиба была выше, чем по данным промежуточного анализа. Не менее одной редукции дозы абемациклиба/плацебо в связи с НЯ потребовалось 46,5% (152) пациенток группы абемациклиба и 6,2% (10) – группы плацебо. Лечение каким-либо из исследуемых препаратов было отменено в связи с НЯ у 25,1% (82) пациенток группы абемациклиба и 4,3% (7) – группы плацебо. Полная отмена лечения по протоколу в связи с НЯ потребовалась 16,5% (54) пациенток группы абемациклиба и 3,1% (5) – в группе плацебо. Отмена лечения абемациклибом/плацебо с продолжением получения НИА в связи с НЯ потребовалась 9,5% (31) пациенток группы абемациклиба и никому в группе плацебо. В группе абемациклиба диарея купировалась модификацией дозы: ее редукция была проведена 16,7% пациенток; пропуск дозы в связи с диареей – 19% больных. Как правило, диарея не влияла на длительность лечения в группе абемациклиба: отмена терапии в связи с диареей потребовалась 1,8% (6) пациенток. У четверых из них до отмены терапии дозу не редуцировали. Следует отметить, что в группе абемациклиба у половины пациенток с отменой лечения в связи с НЯ редукцию дозы до этого не проводили. И наоборот, 74% (112 из 152) пациенток с редукцией дозы в связи с НЯ не потребовалась отмена лечения из-за токсичности [6, 39].
Выводы
- Абемациклиб – избирательный ингибитор CDK4/6 непрерывного перорального применения с активностью в отношении CDK4, в 14 раз выше, чем в отношении CDK6.
- Абемациклиб зарегистрирован в комбинации с эндокринотерапией в качестве первой линии лечения и после прогрессирования на фоне гормонотерапии. Абемациклиб – единственный ингибитор CDK4/6, зарегистрированный в качестве применения в режиме монотерапии после прогрессирования на фоне эндокринотерапии и предшествовавшей химиотерапии по поводу ЭР+ HER2- мРМЖ.
- Особенно важно, что значимая эффективность и удовлетворительная переносимость абемациклиба отмечены у пациенток с ЭР+ HER2- мРМЖ с неблагоприятными про-гностическими факторами: метастазами в печени, отрицательным статусом рецепторов прогестерона, агрессивным течением (ПОЛ<36 месяцев с момента окончания адъювантной эндокринотерапии; короткий период с момента постановки диагноза).


