Введение
Несмотря на существенные успехи в лечении рака молочной железы (РМЖ), заболевание неизлечимо и целями терапии являются продление жизни, когда это возможно, но самое главное – уменьшение симптоматики и улучшение качества жизни [1, 3]. На выбор лечения пациенток с метастастатическим РМЖ (мРМЖ) влияет множество параметров, включая характеристики опухоли, стадию заболевания, варианты предыдущей терапии, возраст, общее состояние, наличие сопутствующих заболеваний, пожелания пациенток и доступность лекарственных препаратов [1, 2]
Роль пероральной химиотерапии в лечении мРМЖ была изучена на двух дискуссионных форумах, проведенных в Сан-Антонио в декабре 2014 и 2015 гг.
Эксперты обсуждали варианты химиотерапии для мРМЖ и потенциальную роль пероральных цитотоксических препаратов в обеспечении эффективной паллиативной помощи.
ABC3 рекомендует выбор лечения, основываясь на таких факторах, как особенности опухоли (статус гормональных рецепторов и экспрессия HER2), общие характеристики (возраст, сопутствующие заболевания, менопаузальный статус) и предпочтения пациентки [1, 2].
Оптимальный выбор, последовательность и продолжительность терапии для пациенток с распространенным РМЖ продолжают обсуждаться. Для трижды негативного РМЖ химиотерапия является основным вариантом лечения [1, 2]. Многочисленные химиотерапевтические препараты продемонстрировали эффективность для пациенток с HER2-отрицательным мРМЖ, включая пероральные цитостатики [3]. Для больных HER2-положительным раком HER2-блокада служит основой лечения. Комбинированное лечение HER2-ориентированными препаратами используется в комбинации с внутривенной/пероральной химиотерапией или эндокринной терапией [1, 2, 4].
Для пациенток с эндокринно-чувствительным, HER2-негативным раком предпочтительна эндокринотерапия как самостоятельно, так и в комбинации с такими препаратами, как эверолимус [1, 2] и палбоциклиб [5]. Тем не менее резистентность к эндокринотерапии остается серьезной проблемой и почти все пациенты получают химиотерапию во время лечения [1, 2]. Химиотерапия должна назначаться при развитии резистентности к эндокринотерапии, при жизнеугрожающих состояний и/или ярко выраженных симптомах заболевания.
Хотя лечение мРМЖ имеет в первую очередь паллиативный характер, стратегия оптимизации терапии и продление жизни остаются важными факторами [1, 3]. мРМЖ довольно чувствителен к цитостатикам, но ответ на лечение зависит от факторов лечения, особенностей пациентки и течения заболевания. Не смотря на то, что продолжительность лечения и ответ часто уменьшаются по мере увеличения числа линий терапии, многие пациентки хорошо реагируют даже на несколько линий химиотерапии [6, 7].
Чрезвычайно важен вопрос об оптимальной последовательности терапии, а также о продолжительности лечения. Мета-анализ и обзор данных рандомизированных клинических исследований продемонстрировали сравнительную эффективность, включая общую выживаемость, для последовательной и комбинированной химиотерапии [8, 9]. Последовательное назначение цитостатиков ведет к снижению количества нежелательных явлений, существенно влияюших на качество жизни пациентки. Комбинированная химиотерапия ассоциируется с более быстрым ответом, поэтому она показана пациенткам с быстро прогрессирующими или угрожающими жизни формами заболевания или симптомными метастазами. Оптимальная последовательность химиотерапии зависит от наличия в анамнезе адъювантной химиотерапии и ее варианта, интервала между линиями терапии и выбора пациентки [1, 3]. Многие пациентки получали в адъюванте антрациклины с таксанами или без них. Хотя пациентки с мРМЖ могут быть повторно пролечены альтернативным режимом на основе антрациклинов, если существует длительный интервал без болезни, предпочтительными считаются другие варианты лечения [1, 2]. Таксаны остаются важной терапевтической опцией первой линии для мРМЖ у пациентов, которые не получали лечения таксанами или у кого прогрессирование заболевания наступило более чем через 12 месяцев после завершения адъювантной терапии другим таксаном.
мРМЖ – неизлечимая болезнь и важно соблюсти баланс борьбы с болезнью и побочными эффектами, которые могут быть связаны с длительным воздействием цитостатиков и ограниченной выживаемостью [3]. Таким образом, важно обсудить все варианты лечения с больными MРМЖ и оценить соотношение риска и пользы, отражающее цели улучшения как продолжительности, так и качества жизни пациентки [1, 3]. Нужды пациентки, ее личные предпочтения и ожидания служат важным фактором при выборе и принятии решения. Сейчас для лечения мРМЖ доступны многочисленные пероральные препараты, в т.ч. цитостатики и таргетные препараты.
По сравнению со стандартной внутривенной химиотерапией пероральная химиотерапия обеспечивает пациенткам больше удобств и позволяет клиницистам легче адаптировать терапию при необходимости [10]. Кроме того, снижается количество времени для нахождения в клинике, что ведет к экономии затрат и времени персонала. Опросы показывают, что большинство пациенток предпочитают пероральную терапию внутривенной, когда установлена их эквивалентная эффективность [11, 12]. Однако при проведении пероральной химиотерапии сохраняются потенциально опасные побочные эффекты и поэтому с точки зрения безопасности важно эффективно обучить пациенток и наблюдать за ними, кроме того, важны и простые режимы дозирования [10]. Приверженность пациентки режиму приема лекарственных препаратов зависит от нескольких факторов, включая сложность схемы лечения, неудобство или неэффективность лечения, отсутствие контакта с медицинскими работниками, побочные эффекты со стороны желудочно-кишечного тракта, отягощенный анамнез психических заболеваний [13, 14]. Неправильное обращение или хранение пероральных препаратов также может быть проблемой и ставить под угрозу эффективность лекарства [15].
Как капецитабин, так и винорелбин продемонстрировали значительную эффективность и переносимость при мРМЖ, особенно в качестве терапии второй и третьей линий после отказа от таксанов. Капецитабин продемонстрировал эффективность в нескольких исследованиях III фазы, в то время как данные по пероральному винорелбину в настоящее время ограничены исследованиями II фазы [16, 17]. Обзор данных более 2000 пациенток, пролеченных антрациклинами и таксанами и получавших монотерапию капецитабином или винорелбином, показали эффективность этих химиотерапевтических препаратов, которые обеспечивают средние показатели контроля заболевания (общий ответ плюс стабилизация) примерно 55 и 50% соответственно [18].
Монотерапия капецитабином показывает беспрогрессивную выживаемость от 3,0 до 7,9 месяца среди пациенток с мРМЖ [19–30]. В недавнем обзоре 31 исследования пероральный винорелбин более чем у 1000 пациенток с мРМЖ продемонстрировал хорошую эффективность и переносимость как в монотерапии, так и в комбинации с капецитабином или с таргетной терапией [17]. В качестве монотерапии пероральный винорелбин ассоциировался со средним значением беспрогрессивной выживаемости от 4,0 до 8,2 месяца [17, 31–36]. Комбинация перорального винорелбина с капецитабином также эффективна и демонстрирует выживаемость в диапазоне от 3,4 до 10,5 месяцев у пациенток с мРМЖ [17, 37–44].
Нет прямого сравнения между использованием этих двух препаратов в комбинации, а не в последовательности. Назначение комбинации препаратов более связано со значительной частотой побочных эффектов и может быть рекомендована, например, пациентам с выраженными симптомами заболевания. На основании имеющихся данных руководство ABC3 рекомендует капецитабин, винорелбин или эрибулин как предпочтительный выбор для пациенток, которые ранее получали антрациклины, таксаны и не требуют комбинированной химиотерапии [1, 2].
В отношении пациенток с HER2-положительным мРМЖ как капецитабин, так и винорелбин продемонстрировали эффективность и переносимость в комбинации с таргетными препаратами. Капецитабин эффективен в комбинации с трастузумабом и лапатинибом со средней продолжительностью жизни 8,2 месяца и 6,2 месяца для пациенток с HER2-положительным мРМЖ после прогрессирования на трастузумабе в первой линии химиотерапии [45, 46]. Внутривенный винорелбин также продемонстрировал эффективность при HER2-положительном заболевании, демонстрируя аналогичные или лучшие, чем таксаны, показатели ответа и продолжительность жизни в сочетании с трастузумабом в качестве химиотерапии первой линии в исследованиях III фазы TRAVIOTA и HERNATA [47, 48]. Кроме того, в ретроспективном исследовании пероральное применение винорелбина в комбинации с трастузумабом оказалось по крайней мере таким же эффективным, как и стандартная комбинация таксанов и трастузумаба [49]. Эффективность винорелбина плюс трастузумаб вместе с хорошей переносимостью делает эту комбинацию важной опцией для лечения HER2-положительного мРМЖ. В исследованиях II фазы получены многообещающие данные по безопа-сности и эффективности комбинации винорелбина с трастузумабом и пертузумабом [50].
Винорелбин (Навельбин) – полусинтетический алкалоид барвинка розового известен с 1987 г. Обладая равнозначной эффектиностью с внутривенными препаратами, меньшим спектром побочных эффектов и удобством приема, пероральный винорелбин стал важным инструментом в руках химиотерапевта. Основной механизм действия винорелбина – блокировка митоза клеток на стадии метафазы за счет действия на митотические трубочки.
Крайне интересно использование перорального винорелбина при мРМЖ в реальной клинической практике.
Клинический случай
Пациентка Б. 66 лет. Больна с 2010 г.
Цитология от 24.06.2010: умеренно дифференцированный протоковый канцер. Диагноз на момент выявления первичной опухоли: рак левой молочной железы ст IIIB T3N2M0 гр II ИГХ Эр+ H110 Пр+ H120 Her2neu ++ Ki-67 80%.
Пациентке проведено 5 курсов неоадъювантной полихимиотерапии в режиме 4АС-4D (100 мг/м²) (c июля по ноябрь 2010 г.). В декабре 2010 г. проведена дистанционная лучевая терапия на молочную железу, затем мастэктомия слева (23.01.2011). Гистологически после операции: инфильтрирующая протоковая карцинома 3-й степени лечебного патоморфоза. В послеоперационном периоде пациентке назначена адъювантная гормонотерапия тамоксифеном 20 мг/сут, прием которого она продолжала до 2015 г. В октябре 2017 г. отметила появление одышки, боли в грудной клетке справа. По данным компьютерной томографии (КТ) органов грудной клетки от 06.11.2017: по всем полям паренхимы правого легкого по ходу костальной и междолевой плевры визуализируются множественные очаги от 6×37 до 14×15 мм. По задней поверхности правого легкого определяется выпот до 97,8 мм. Лимфоузлы средостения увеличены до 10×9 мм (рис. 1). Констатирова-но прогрессирование заболевания. Начат курс перорального винорелбина еженедельно с 29 ноября 2017 г.: 60 мг/м2 – 120 мг 1-й, 8-й, 15-й дни, затем по 80 мг/м2 – 160 мг 1 раз в течение 7 дней, всего 12 недель до КТ-контроля. Лече-ние переносилось хорошо, побочных эффектов не наблюдалось.
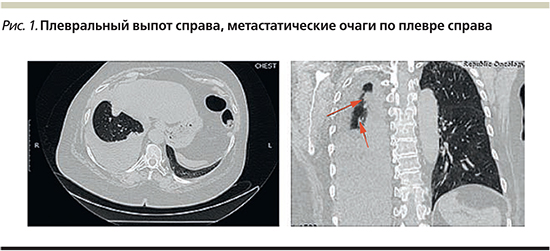
КТ-контроль 14.03.2019: в правой плевральной полости определен уровень жидкости до 75 мм. В правом легком разнокалиберные участки по плевре и субплеврально размером от 3 до 15 мм. Лимфоузлы не увеличены. Общая картина соответствует стабилизации с положительными эффектами по лимфоузлам средостения и размером легочных очагов. Терапия винорелбином продолжена с 04.04.2018: 160 мг перорально 1 раз в 7 дней в течение 12 недель. Терапию перенесла удовлетворительно, негематологической и гематологической токсичности не наблюдалось. Контроль КТ 09.07.2018: по плевре справа уплотнения размером 3–10 мм. Уровень плеврального выпота – 65 мм (рис. 2).
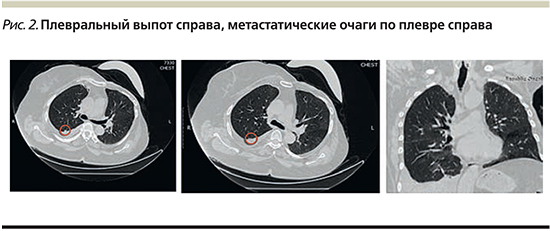
Терапию проводили в течение 24 недель, на фоне лечения уровень одышки постепенно снизился, боли полностью прекратились. Статус ECOG (Eastern Cooperative Oncology Group) колебался от 0 до 1. После достижения ремиссии пациентка переведена на поддерживающую терапию фулвестрантом 500 мг внутримышечно 1 раз в 28 дней. Получает препарат по настоящее время с июля 2018 г. без прогрессирования. Таким образом, применение перорального навельбина является прекрасной терапевтической опцией, характеризующейся высокой эффективностью при низком профиле токсичности.
Заключение
Баланс между эффективностью и качеством жизни очень важен для пациенток с мРМЖ [1, 2]. Выбор химиотерапии, эндокринной терапии и таргетных препаратов должен основываться на текущих рекомендациях по лечению, данных клинических исследований, тщательного анализа характеристик пациентки и заболевания, и, что очень важно, – на предпочтениях пациентки. Последовательная монотерапия является предпочтительной опцией для подавляющего большинства пациенток, которым требуется химиотерапия. Химиотерапию следует продолжать до прогрессирования заболевания и до тех пор, пока она хорошо переносится.
В рамках вариантов лечения интерес вызывает использование препаратов пероральной химиотерапии, таких как капецитабин и пероральный винорелбин [10]. Эти препараты позволяют обеспечивать контроль болезни, обладают хорошей переносимостью и уменьшают время и затраты, связанные с лечением. Тем не менее инструктаж пациенток имеет основополагающее значение для обеспечения надлежащего безопасного использования перорального химиотерапевтического препарата [10, 13]. Пероральная химиотерапия – хороший вариант для поддерживающего лечения и для продления контроля заболевания.



