Введение
Астенический синдром (АС) встречается в практической деятельности врачей всех специальностей (терапевтов, эндокринологов, кардиологов, психиатров), во всех возрастных группах пациентов. На неврологическом приеме о признаках астении сообщают более половины больных, причем частота и распространенность АС имеют тенденцию к неуклонному росту, особенно среди групп населения молодого и среднего возраста [1–3].
Определение АС в Международной классификации болезней 10-го пересмотра (МКБ-10) сформулировано как «постоянное ощущение и/или жалобы на чувство общей слабости, повышенной утомляемости (при любом виде нагрузки), а также снижение работоспособности, которые сочетаются с 2 или более из нижеперечисленных жалоб: мышечные боли, головные боли напряжения, головокружение, нарушения сна, диспепсия, неспособность расслабиться, раздражительность», т.е. сделан акцент на общую слабость и повышенную утомляемость как на ключевые признаки астении. Утомляемость и усталость в англоязычной литературе обозначаются одним словом fatigue, в отечественной литературе усталость и утомляемость имеют различное смысловое значение. Утомляемость – это уменьшение работоспособности в ходе выполнения какой-либо физической или умственной нагрузки, а усталость – это субъективное ощущение утомляемости [3]. Усталость может иметь физиологический характер, возникая в результате истощения энергетических запасов у здоровых лиц после нагрузки и исчезая после отдыха, и патологический – когда возникает вне связи с нагрузкой и сохраняется после отдыха, являясь следствием нарушения регуляции использования энергетических ресурсов. Патологическая слабость и утомляемость при астении имеют характерные особенности: возникают и при нагрузке, и без нее и не исчезают после отдыха.
Общепринятой классификации астении в настоящее время не существует, взгляды на патогенез АС также противоречивы. В МКБ-10 астения может быть отнесена к классу «Невротические, связанные со стрессом и соматоформные расстройства» (F4) в рубрике «Неврастения» или к классу «Симптомы, признаки и отклонения от нормы, выявленные при клинических и лабораторных исследованиях, не классифицируемые в других группах» (R13) в рубрике «Недомогание и утомляемость» (R53). Астения, возникшая после перенесенной новой коронавирусной инфекции, может быть рубрифицирована как «Синдром усталости после перенесенной вирусной инфекции» (G93.3) или описана в структуре постковидного синдрома «Состояние после COVID-19» (U09.9).
В отечественной литературе и клинической практике традиционно используется понятие «астения» или «астенический синдром», в англоязычной литературе более распространен термин «синдром хронической усталости» – СХУ (CFS – Chronic Fatigue Syndrome). Критерии диагностики СХУ неоднозначны, существуют разногласия по поводу этиологии, патофизиологии, наименования, числа необходимых симптомов для установления диагноза и подходов к лечению [4, 5]. По критериям диагностики СХУ (2015) для постановки диагноза требуется три симптома и по крайней мере одно из двух дополнительных проявлений: 1) значительное снижение или ухудшение способности заниматься обычной профессиональной, образовательной, социальной активностью, которое длится более 6 месяцев, сопровождается усталостью, которая не является результатом продолжительной или необычной чрезмерной нагрузки, причем отдых существенно не облегчает состояние; 2) недомогание после физической нагрузки, умственного или эмоционального напряжения, которое не вызывало проблемы до болезни, симптомы обычно усиливаются через 12–48 часов после активности или воздействия и могут продолжаться в течение нескольких дней или даже недель; 3) не освежающий сон – пациенты могут не чувствовать себя лучше или менее уставшими даже после полноценного ночного сна. Должно присутствовать по крайней мере одно из следующих двух дополнительных проявлений: 1) когнитивные нарушения (снижение памяти, исполнительных функций и обработки информации, дефицит внимания и нарушения психомоторных функций), усугубляющихся при стрессе, напряжении, длительном пребывании в вертикальном положении; 2) ортостатическая непереносимость – ухудшение симптомов при принятии и поддержании вертикального положения (обмороки или головокружение, головные боли, тошнота, усиливающиеся при спокойном вертикальном положении и улучшающиеся в положении лежа. Дополнительные симптомы СХУ включают различные болевые синдромы: боль в мышцах, в суставах, головные боли, боль в горле, болезненные лимфатические узлы на шее или подмышечной впадине [4]. СХУ в МКБ-10 кодируется как «Синдром утомляемости после перенесенной вирусной болезни. Доброкачественный миалгический энцефаломиелит» (G93.3) [6].
Большинство авторов в настоящее время выделяют астению первичную, вторичную (соматогенную, органическую, симптоматическую) и реактивную. Первичная астения рассматривается, как правило, в качестве самостоятельной клинической единицы, ведущими симптомами которой являются постоянное ощущение слабости и патологическая утомляемость, которые приводят к физической и социальной дезадаптации и не могут быть объяснены другими причинами (инфекционными, соматическими, психическими заболеваниями или токсическими факторами). Астения вторичная (органическая, соматогенная) возникает у пациентов, имеющих хроническую соматическую патологию (заболевания печени и почек, эндокринную и метаболическую патологию, туберкулез, ВИЧ, онкологические, нейродегенеративные заболевания, последствия инвалидизирующих травм). Реактивная астения наиболее часто встречается среди всех форм астении, в ее формировании ключевая роль отводится психофизическим перегрузкам во время работы, нарушениям режима труда и отдыха (например, при сменном графике работы), выраженным стрессовым ситуациям. К факторам риска развития реактивной астении относятся работа в сменном режиме, частая и быстрая смена часовых поясов, длительное повышенное напряженное внимание во время трудовой деятельности или частое переключение внимания в условиях эмоционального напряжения (например, у синхронных переводчиков, авиадиспетчеров), эмоциональные перегрузки. Реактивная астения возникает у здоровых лиц в условиях воздействия неблагоприятных факторов, вызывающих дезадаптацию (при сезонных авитаминозах, строгом соблюдении низкокалорийной диеты, при длительном ежедневном контакте с гаджетами, после хирургических операций, инфекционных заболеваниях, при напряженной ответственной работе в течение длительного времени, без отпусков, без эмоциональной и физической разгрузки). Астения может возникать у школьников и студентов при значительных умственных и эмоциональных нагрузках, например в период экзаменационной сессии или при сочетании напряженной учебы и трудовой деятельности [7–9].
Ведущее значение в развитии астении принадлежит нарушению функции восходящей ретикулярной активирующей системы ствола мозга, обеспечивающей поддержание уровня внимания, восприятия, бодрствования и сна, вегетативной регуляции, общей и мышечной активности. Это объясняет полиморфизм АС, включающего не только утомляемость и слабость, но и эмоциональные расстройства, когнитивные симптомы, вегетативную дисфункцию, болевые расстройства. Эмоциональные расстройства при астении характеризуются раздражительной слабостью: лабильностью настроения с раздражительностью, гневливостью, недовольством окружающими, капризностью и слезливостью. При преобладании в клинической картине раздражительности, возбудимости, обидчивости выделяют гиперстенический вариант АС, характеризующийся сверхвозбудимостью сенсорного восприятия с повышенной восприимчивостью к нейтральным в норме внешним раздражителям (например, непереносимость звуков, света и др.). При преобладании безынициативности, апатии, истощаемости, снижении речевой и двигательной активности, слезливости выделяют гипостенический АС, основным элементом которого является снижение порога возбудимости и восприимчивости к внешним стимулам с повышенной слабостью, вялостью, дневной сонливостью [10].
Для астении характерно нарушение цикла «сон–бодрствование»: затруднение засыпания с неглубоким поверхностным сном, частыми пробуждениями, бессонница ночью и сонливость днем. Типичны для АС соматовегетативные нарушения: тахикардия, лабильность артериального давления, нарушения терморегуляции, гипергидроз, гиперемия лица, холодные бледные конечности, полиурия, метеочувствительность. Для пациентов с АС свойственны и когнитивные нарушения (снижение концентрации внимания и умственной работоспособности, нарушения кратковременной памяти, трудности в воспроизведении ранее усвоенной информации), психологические (снижение мотивации, отсутствие уверенности в себе), сексуальные (снижение эрекции и отсутствие либидо) и болевые расстройства (головные боли напряжения, миалгии, кардиалгии, абдоминалгии, дорсалгии).
Терапия АС должна проводиться в соответствии с этиологическими факторами и основными клиническими проявлениями. Тактика врача при вторичной астении должна быть направлена на лечение основного заболевания и купирование токсико-метаболических расстройств, которые обусловливают развитие астении. В терапию первичной астении включают психотерапевтические методы, физические тренинги и применение различных фармакологических препаратов. При реактивной астении особое внимание необходимо уделять коррекции факторов, приведших к появлению дезадаптации, объяснять пациенту механизмы возникновения ее симптомов и рекомендовать нормализовать режим труда и отдыха, сна и бодрствования (нормированный рабочий день, нормальные условия трудового процесса, адекватный сон, минимизация гипоксии, гиподинамии, борьба с приемом алкоголя и табакокурением) [11, 12].
В настоящее время коррекция астенических проявлений продолжает оставаться сложной медицинской проблемой, пациентам может быть назначен сразу целый комплекс лекарственных средств, направленных на уменьшение многообразных астенических проявлений: антидепрессанты, нейролептики, вегетотропные средства, витамины, анксиолитики, адаптогены, биологически активные добавки. По современным представлениям о патогенезе клинических проявлений АС, наиболее перспективным в терапии является воздействие на ядро астенических расстройств – гипоэргоз с повышенной истощаемостью психических функций.
Современная нейрометаболическая энергокоррекция может способствовать повышению устойчивости и жизнеспособности нейронов, устранять нейробиохимические нарушения, которые наблюдаются при астении. Активное использование при лечении АС находят препараты, фармакологические свойства которых связаны с полимодальным действием. Представителем данной группы является препарат фонтурацетам (Актитропил, ОАО «Фармстандарт-Лексредства», Россия) – ноотропный препарат (код АТХ: N06BX – Другие психостимуляторы и ноотропные препараты) [13]. Препарат оказывает положительное влияние на обменные процессы и кровообращение мозга, стимулирует окислительно-восстановительные процессы, повышает энергетический потенциал организма за счет утилизации глюкозы. Фонтурацетам как ноотропный препарат оказывает прямое активирующее влияние на интегративную деятельность головного мозга, повышает скорость передачи информации между полушариями головного мозга, способствует консолидации памяти, улучшает концентрацию внимания и умственную деятельность, регулирует процессы активации и торможения центральной нервной системы. Стимулирующее действие фонтурацетама проявляется в его способности оказывать умеренно выраженное действие в отношении двигательных реакций, в повышении физической работоспособности; психостимулирующее действие преобладает в идеаторной сфере. Адаптогенное действие фонтурацетама проявляется в повышении устойчивости организма к стрессу в условиях чрезмерных психических и физических нагрузок, при утомлении, причем действие проявляется с однократной дозы, что важно при применении препарата в экстремальных условиях. Умеренный психостимулирующий эффект препарата сочетается с анксиолитической активностью, улучшает настроение, повышая порог болевой чувствительности. При курсовом применении фонтурацетама не развиваются лекарственная зависимость, толерантность, «синдром отмены», препарат не обладает тератогенными, мутагенными, канцерогенными и эмбриотоксичными свойствами.
Цель исследования: изучить клиническую эффективность и безопасность применения препарата Актитропил для пациентов молодого возраста с реактивным АС.
Материал и методы
В открытое наблюдательное проспективное несравнительное нерандомизированное исследование были включены 50 пациентов (29 женщин и 21 мужчина) в возрасте от 18 до 30 лет (средний возраст – 24,7±5,4 года) с клиническими проявлениями реактивной астении, находившихся на амбулаторном лечении и давших письменное информированное согласие на участие в клиническом наблюдении.
Критерии включения: наличие астенического состояния как ведущего проявления болезни по субъективной шкале оценки астении MFI-20 (Multidimensional Fatigue Inventory – многомерный опросник по утомляемости) с общим баллом по шкале MFI-20 >30 и длительностью заболевания от 2 до 6 месяцев, отсутствие у пациентов текущих острых соматических заболеваний.
Критерии невключения в исследование: соматические заболевания в стадии обострения и декомпенсации, тяжелые психические отклонения, онкологические заболевания, острые инфекционные заболевания, противопоказания к применению препарата в соответствии с инструкцией (повышенная чувствительность к любому из компонентов препарата, возраст младше 18 лет, беременность, период грудного вскармливания).
Актитропил назначался в соответствии с инструкцией по зарегистрированным показаниям: заболевания центральной нервной системы, сопровождающиеся ухудшением интеллектуально-мнестических функций, снижением двигательной активности; нарушением внимания, ухудшением памяти. Во время курса лечения фонтурацетамом пациентам не назначались другие ноотропные и антиастенические препараты, витаминотерапия, а также нейропротективные, антиоксидантные, анксиолитические и антиастенические препараты, препараты, улучшающие микроциркуляцию и метаболизм головного мозга. Пациенты получали монотерапию Актитропилом в дозе 200 мг/сут: 100 мг утром (1 таблетка) и 100 мг днем (до 15 часов), препарат назначался после приема пищи. Длительность терапии составила 30 дней.
Клиническое наблюдение проводилось в течение 60 дней и включало 3 визита: 1-й визит – принятие решения о включении пациента в программу до начала приема фонтурацетама; 2-й визит – завершение лечения, на 30-й день после начала терапии для оценки влияния препарата на динамику симптомов астении; 3-й визит – анализ отсроченного эффекта фонтурацетама в отношении симптомов астении на 60-й день после начала терапии. До назначения препарата каждому пациенту проводилось стандартное клинико-неврологическое исследование для постановки диагноза, включающее сбор медицинского анамнеза, общеклинический и неврологический осмотры, а также заполнялись диагностические опросники. Во время каждого визита собирались данные по шкалам клинической оценки, регистрировались побочные эффекты и прием сопутствующих препаратов, все результаты вносились в информационно-регистрационную карту пациента.
Все пациенты жаловались на разнообразные проявления АС от 3 до 6 месяцев, развитию реактивной астении предшествовал психоэмоциональный стресс, физическое перенапряжение, нарушения в организации труда (ненормированный рабочий день, работа в ночное время, неадекватные психоэмоциональные перегрузки, работа без отпусков). Пациенты отрицали в анамнезе хронические соматические заболевания.
Для получения субъективной количественной оценки общей тяжести астении использована шкала оценки астении MFI-20 (The Multidimensional Fatigue Inventory, разработанная группой нидерландских ученых E. Smets, B. Garssen, B. Bonke, J. Haes в 1994 г.), которая в многочисленных исследованиях пациентов с астеническими состояниями различной этиологии показала свою надежность и валидность. Шкала состоит из 20 утверждений, которые отражают разные составляющие астении по 5 субшкалам: общая астения, физическая астения, пониженная активность, снижение мотивации и психическая астения. Оценка субшкалы может варьироваться в интервале от 4 до 20 баллов. Клинически выраженная астения считается при превышении 12 баллов по любой субшкале и при общей сумме баллов более 60 по всем субшкалам [14]. Оценка по шкале MFI-20 проводилась на визитах 1–3.
Для оценки функционального психоэмоционального состояния применяли опросник по самочувствию, активности, настроению (САН; разработан В.А. Доскиным, Н.А. Лаврентьевой, В.Б. Шараем, М.П. Мирошниковым в 1973 г.), который состоит из 30 пар противоположных характеристик, по которым испытуемого просят оценить свое состояние. Средний балл шкалы равен 4. Оценки, превышающие 4 балла, свидетельствуют о благоприятном состоянии испытуемого, ниже 4 – о неблагоприятном состоянии. Нормальные оценки состояния располагаются в диапазоне 5,0–5,5 балла [15, 16]. Оценка по шкале САН проводилась на визитах 1–3.
Выраженность психоэмоциональных нарушений оценивали по госпитальной шкале оценки тревоги и депрессии HADS (Hospital Anxiety and Depression Scale), которая позволяет получать количественную оценку субъективной тяжести тревожных и депрессивных нарушений при подсчете баллов по вопросам, характеризующим данные расстройства в отдельности. Показатели 0–7 баллов – отсутствие достоверно выраженной тревоги/депрессии, 8–10 баллов – субклинически выраженная тревога/депрессия, 11 баллов и более – клинически выраженная тревога/депрессия [17]. Оценка по шкале HADS проводилась на визитах 1–3.
Для оценки качества сна использовалась анкета «Шкала оценки субъективных характеристик сна Шпигеля»: оценивали длительность времени засыпания, продолжительность сна, число ночных пробуждений, частоту сновидений. Показатели, соответствующие здоровому сну, – более 22 баллов, умеренные нарушения сна – от 12 до 22, выраженные – менее 12 баллов [18]. Оценка по шкале Шпигеля проводилась на визитах 1–3.
Эффективность лечения оценивали по шкале глобального клинического воздействия (CGI-I) после лечения (оценка субъективного мнения врача-исследователя с мнением: очень хорошее улучшение, хорошее улучшение, минимальные улучшения, нет изменений, минимальное ухудшение). Оценку эффективности лечения по шкале общего впечатления пациента проводили по градациям: резко улучшилось, улучшилось, улучшилось незначительно, мало изменилось с момента начала исследования [19]. Оценка по шкале CGI-I проводилась на визите 2.
Для оценки безопасности применения препарата определяли наличие побочных эффектов на основании жалоб пациентов и результатов общего осмотра.
Результаты исследования были обработаны с помощью компьютерных программ SPSS и Statistica. Достоверность различий принималась при p<0,05.
Результаты
Во время 1-го визита пациенты предъявляли жалобы на выраженную слабость, быструю утомляемость при физической и умственной работе, повышенную психоэмоциональную истощаемость, снижение жизненного тонуса, вялость, эмоциональную неустойчивость, забывчивость, уменьшение концентрации внимания и памяти, нарушения сна (недостаточную продолжительность сна – менее 6 часов), дневную сонливость, разнообразные болевые ощущения различной локализации и интенсивности. Перечисленные жалобы возникали в течение дня и не проходили после отдыха. У пациентов отмечалась повышенная чувствительность к внешним раздражителям и к собственным ощущениям, что отражалось в многочисленных соматических жалобах. Патологии в неврологическом статусе не выявлялось. Соматический осмотр выявил колебания артериального давления дистонического характера, смешанный дермографизм. На этапе 1-го визита до начала терапии фонтурацетамом среднее значение общего балла по шкале MFI-20 составило 76 (при нормальном показателе 20–30 баллов) и по каждой из субшкал – более 12 баллов, что считается значимым для постановки диагноза АС. Анкетирование показало, что выраженность АС была максимальной по субшкалам «общая астения», «физическая астения» и «психическая астения».
Все пациенты, принимавшие курсовое лечение в течение 30 дней фонтурацетамом, отмечали уменьшение слабости и утомляемости, появление бодрости, активности, ощущение «прилива сил», стремления к деятельности, нормализацию сна, исчезновение дневной сонливости и улучшение настроения. При оценке выраженности АС по шкале MFI-20 (2-й визит) отмечено, что достоверно уменьшились суммарный балл и показатели астении по субшкалам общей астении, психической астении, снижения активности, физической астении и снижения мотивации. В отсроченном периоде в последующий месяц после прекращения лечения (3-й визит) снижение показателей по общей шкале и всем субшкалам продолжалось (табл. 1).
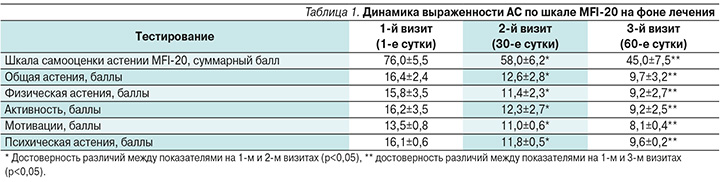
Статистически значимые положительные сдвиги свидетельствовали об эффективном и устойчивом антиастеническом действии препарата.
При анализе функционального психоэмоционального состояния пациентов по данным шкалы САН выявлено статистически значимое повышение медианы значений баллов по отдельным субшкалам через 30 дней после начала лечения и в отсроченном периоде (p<0,05 для всех показателей). Сопоставление оценок тревоги и депрессии по шкале HADS в динамике на 30-й день от начала лечения по сравнению с исходными данными и в отсроченном периоде показало значимое улучшение (p<0,05 для обоих показателей). При анализе качества сна на исходном уровне выявлены умеренные нарушения со средним показателем по шкале Шпигеля 16,9±2,1; причем показателей, соответствующих здоровому сну (более 22 баллов) и выраженным нарушениям сна (менее 12 баллов) зарегистрировано не было, все результаты находились в диапазоне от 22 до 12 баллов. Через 30 дней после начала лечения показатель увеличился и в отсроченном периоде достиг 22 баллов, что соответствует характеристике здорового сна (табл. 2).

Эффективность лечения оценивали с использованием шкал глобального клинического воздействия (CGI-I) после лечения по оценке субъективного мнения врача-исследователя и пациента. Оценка субъективного мнения врача-исследователя после лечения распределилась следующим образом: очень хорошее улучшение – 76%, хорошее улучшение – 18%, минимальные улучшения – 6%, нет изменений – 0, минимальное ухудшение – 0. Оценка по шкале общего впечатления пациента после окончания лечения: сильно улучшилось с момента исследования – 72%, улучшилось с момента начала исследования – 20%, улучшилось незначительно с момента начала исследования – 8%, мало изменилось с момента начала исследования – 0.
Препарат хорошо переносился пациентами, и ни в одном из случаев не потребовалось изменения схемы дозирования. Побочных эффектов, связанных с приемом препарата Актитропил, которые могли бы стать причиной прекращения лечения, также зарегистрировано не было.
Выводы
- Использование препарата Актитропил в дозе 200 мг/сут в 2 приема в течение 30 дней позволяет достигать значительного антиастенического эффекта, активизирует процессы психоэмоциональной и психофизической адаптации в условиях психических и физических перегрузок. Монотерапия фонтурацетамом улучшает эмоциональное состояние пациентов, что позволяет не назначать дополнительные психотропные препараты, тем самым снижая общую медикаментозную нагрузку на пациента.
- Антиастенический и анксиолитический эффекты фонтурацетама сохраняются через месяц после окончания приема препарата.
- Препарат обладает благоприятным профилем безопасности (отмечена хорошая переносимость препарата и отсутствие побочных эффектов на фоне курса лечения).
Таким образом, Актитропил в дозе 200 мг/сут в 2 приема в течение месяца может быть рекомендован для лечения АС, в частности коррекции клинических проявлений реактивной астении у лиц молодого возраста, в качестве монотерапии.



