Введение
Борьба с болью – одна из важнейших задач в работе врача любой специальности. Боль у пациента сопряжена с физическими страданиями и негативными эмоциональными переживаниями. Интенсивная боль вызывает комплекс дезадаптивных реакций в организме. В первую очередь это относится к пациентам со злокачественными новообразованиями, особенно III и IV стадий онкологического процесса. В работах различных авторов отмечается, что хроническая боль занимает ведущее место в перечне тягостных симптомов, от которых страдают пациенты с онкологической патологией [1–4]. Наиболее эффективными болеутоляющими средствами при интенсивной острой и хронической боли, по-прежнему, остаются алкалоиды опия и их синтетические аналоги (опиоидные анальгетики). При хронической боли сильные опиоиды назначают на второй (в низких дозах) и третьей ступенях обезболивания «анальгетической лестницы» Всемирной организации здравоохранения (ВОЗ) [5]. Этот подход одобрен и рекомендован ведущими специалистами по лечению боли [6–13]. К сожалению, группа опиоидных анальгетиков достаточно редко пополняется новыми лекарственными средствами. Классические препараты группы, например морфин и оксикодон, были открыты и применяются очень давно. Морфин был выделен Сертернером в 1804 г., а оксикодон синтезирован в 1916 г. [14].
Новой молекулой, внедренной в широкую клиническую практику в группе опиоидных анальгетиков, стал тапентадол. Препарат был создан как альтернатива слабому опиоиду трамадолу с учетом плюсов и минусов последнего, проявившихся за годы его применения в клинической практике. Напомним, что молекулу трамадола в 1962 г. синтезировали ученые немецкой компании Grunenthal во главе с Хельмутом Бушманном [15]. Трамадол обладает весьма слабым опиоидным действием (аффинитетом в отношении опиатных рецепторов), которое усиливается двумя другими анальгетическими механизмами – ингибированием обратного захвата серотонина и норадреналина. Модификация молекулы трамадола привела к созданию на ее основе тапентадола – сильного анальгетика с улучшенными фармакологическими свойствами. Тапентадол по сравнению с трамадолом имеет более выраженный аффинитет к опиоидным рецепторам, ингибирует обратный захват норадреналина, но мало влияет на серотонинергическую систему и не метаболизируется на ферментах – цитохромах, в частности на 2D6 [16]. Последнее делает метаболизм препарата независимым от генетических особенностей пациента (ультрабыстрые и/или медленные метаболизаторы на цитохроме 2D6, и не приводит к фармакокинетическим лекарственным взаимодействиям с препаратами – индукторами или ингибиторами цитохрома 2D6. Сниженная серотонинергическая активность уменьшает риски развития «серотонинового синдрома» при применении тапентадола. На момент публикации препарат применяется 15 лет в условиях реальной клинической практики. В 2008 г. тапентадол получил одобрение FDA (Food and Drug Administration) для клинического применения и поступил на рынок США. В 2010 г. тапентадол был одобрен для применения в Европе. В 2011 г. в США для лечения умеренной и сильной хронической боли была зарегистрирована лекарственная форма тапентадола с пролонгированным высвобождением. В 2012 г. препарат получил одобрение FDA для лечения невропатической боли (НБ), связанной с диабетической периферической полиневропатией. В России тапентадол был зарегистрирован в 2014 г. под коммерческим названием «Палексия». В России зарегистрированы таблетки с быстрым высвобождением препарата в дозе 50 мг, 75, 100 мг и таблетки пролонгированного действия в дозах 50 мг, 100, 150, 200 и 250 мг. Тапентадол входит в перечень препаратов для бесплатного льготного отпуска пациентам во многих регионах России, в частности, в 2022 г. он включен в региональную льготу в Санкт-Петербурге. Препарат входит в Клинические рекомендации Минздрава по терапии хронической боли у взрослых пациентов паллиативного профиля. В них отмечается, что он применяется онкологическими пациентами в рамках паллиативной помощи при боли умеренной и высокой интенсивности. Суточная доза тапентадола при лечении боли умеренной интенсивности составляет 100 мг.
При боли высокой интенсивности назначается суточная доза тапентадола до 500 мг в таблетках пролонгированного действия.
Особенности фармакологии тапентадола
Фармакологическое действие тапентадола сочетает агонизм в отношении мю-опиоидных рецепторов и ингибирование обратного захвата норадреналина. Механизм воздействия на мю-опиоидные рецепторы прерывает пре- и постсинаптическую передачу восходящих болевых сигналов в спинном мозге и активирует нисходящий тормозной тонус супраспинально. Ингибирование обратного захвата норадреналина увеличивает его содержание в синапсе и усиливает нисходящий тормозной импульс в рамках антиноцицептивной системы организма [17–19]. Это сочетание механизмов отличает препарат от других сильных опиоидов и делает его молекулой нового класса анальгетиков центрального действия, называемых бифункциональными лигандами [20]. В исследованиях in vitro показано, что сродство тапентадола с мю-рецепторами примерно в 50 раз меньше, чем у морфина. Однако его анальгетическая активность в экспериментах на животных с использованием моделей боли была только в 2–3 раза ниже по сравнению с морфином. Экспериментальные данные демонстрируют, что ингибирование обратного захвата норадреналина является механизмом, очень важным при лечении НБ, и он может преобладать над опиоидергическим механизмом [21]. Синергизм двух механизмов анальгетического эффекта приводит к повышению эффективности, снижению дозы опиоида и к уменьшению частоты опиоид-ассоциированных нежелательных эффектов [22–24].
В клинической практике соотношение анальгетической дозы тапентадола к морфину составляет 2,5 к 1, т.е. 250 мг тапентадола по анальгетической активности у пациента, страдающего от боли, эквивалентны эффективности 100 мг морфина для приема внутрь [25].
Исследования анальгетической эффективности тапентадола у онкологических пациентов
С момента начала клинического применения тапентадола выполнено достаточно много исследований анальгетической эффективности препарата у онкологических больных. Работы, выполненные с января 2005 по июль 2015 г., вошли в Кокреновский обзор [26]. В исследованиях, вошедших в обзор, приняли участие 1029 человек. Препарат сначала титровали для определения максимальной эффективной и переносимой дозы, затем проводили поддерживающее лечение. Препарат тапентадол принимался дважды в сутки, дозы варьировались от 50 до 500 мг в сутки. В качестве показателей эффективности использовали численные рейтинговые оценки боли, общее впечатление пациента об изменениях самочувствия и количество использованных пациентами дополнительно обезболивающих препаратов для лечения «прорывов боли». Велся учет нежелательных эффектов и отмен тапентадола. По результатам обзора сделано заключение: тапентадол в эквивалентных дозах имел аналогичные с морфином и оксикодоном анальгетический эффект и переносимость.
Одним из основных исследований, вошедших в этот обзор, было рандомизированное плацебо-контролируемое двойное слепое исследование эффективности и переносимости тапентадола пролонгированного действия (ПД) по сравнению с плацебо и морфином при лечении умеренной и сильной онкологической боли [27]. Результаты исследования показали, что тапентадол ПД является эффективным препаратом для лечения умеренной и интенсивной боли (ноцицептивной и невропатической) при злокачественных новообразованиях. По эффективности он не уступает морфину, но обладает лучшей переносимостью со стороны желудочно-кишечного тракта (ЖКТ). В позднее опубликованном обзоре этого автора было констатировано, что тапентадол был исследован в различных группах онкологических больных [28]. Пациенты страдали болью смешанной этиологии и патогенеза, в т.ч. болью при гематологических злокачественных новообразованиях и болью, развивавшейся из-за противоопухолевого лечения. Во всех группах пациентов тапентадол по эффективности обезболивания не уступал морфину или оксикодону. Автор отметил, что сочетание эффективности тапентадола с общим благоприятным профилем безопасности делает его предпочтительным для лечения онкологических больных, страдающих не только от боли, но и от тошноты, рвоты, запоров или других нежелательных эффектов, типичных для опиоидов и снижающих качество их жизни. В обзоре
А. Carmona-Bayonas et al. проанализированы исследования, размещенные в PubMed, Кокрановской библиотеке, EMBASE и Google Scholar с 2008 по 2016 г., посвященные применению тапентадола у онкологических больных при умеренной или сильной боли [29]. Проанализированные исследования показали, что тапентадол хорошо переносится и является эффективным средством при онкологических болях умеренной и высокой интенсивности. Авторы сделали выводы: тапентадол может быть альтернативой классическим опиоидам при раке, отличаясь от них лучшей переносимостью. В одном из последних опубликованных обзоров также было констатировано, что тапентадол продолжает в новых рандомизированных исследованиях демонстрировать эффективность, идентичную морфину и оксикодону [30]. При этом препарат эффективен в отношении как «опиоиднаивных» пациентов, так и тех, кто принимал опиоиды до использования тапентадола. Было подчеркнуто, что отличительной чертой тапентадола несомненно является высокая эффективность при НБ различного генеза, в частности, развивающаяся у онкологических пациентов.
Эффективность применения тапентадола у онкологических пациентов с НБ
При НБ происходит функциональное подавление или десенсибилизация мю-опиоидных рецепторов в дорсальном роге спинного мозга [31], также нарушается функция нисходящей норадренергической тормозной антиноцицептивной системы. Причем второе является даже более значимым фактором [32]. Причины развития НБ у онкологических пациентов могут быть разнообразными. Это НБ, вызванная непосредственно ростом опухоли (66% случаев), боль, связанная с применяемыми методами лечения (хирургическое вмешательство, химиотерапия с применением таксанов, препаратов на основе платины, алкалоидов барвинка, талидомида, ингибиторов протеасом, радиационно-индуцированная невропатия). Она наблюдается примерно в 20% случаев. Также НБ может вызываться заболеваниями, сопутствующими онкологическому процессу (10–15% случаев) [33, 34].
В рамках этих причин, механизмы развития НБ у онкологических больных могут быть различными [35]. Они представлены в таблице.
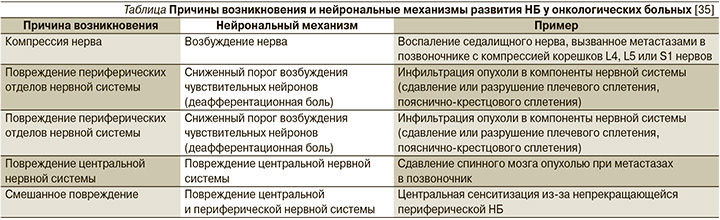
В российских клинических рекомендациях по лечению НБ в случае, если она связана с развитием онкологического процесса, предлагается использовать для лечения опиоидные препараты (трамадол, морфин, оксикодон, тапентадол), согласно «лестнице обезболивания ВОЗ», как альтернативу или дополнение к терапии трициклическими антидепрессантами (амитриптилин) и антиконвульсантами (габапентин, прегабалин) [36].
В настоящее время проведены различные исследования, подтвердившие эффективность тапентадола при НБ, возникающей по тем или иным причинам при онкологических заболеваниях.
В ретроспективном одноцентровом открытом нерандомизированном исследовании 38 пациентов с раком в далеко зашедшей стадии, которые исходно получали опиоиды, такие как трамадол, пероральный оксикодон, трансдермальный фентанил, с адъювантными анальгетиками или без них, но не отмечали достаточного эффекта, после перехода на тапентадол, боль уменьшилась. Был сделан вывод: тапентадол эффективно облегчает НБ при раке, которая была мало восприимчива к лечению ранее назначенными опиоидами [37]. Еще в одном исследовании действие тапентадола на НБ оценивали у 40 онкологических пациентов, которые получали тапентадол с июня 2017 по май 2020 г. [38]. Показатель оценки боли по нумерологической рейтинговой шкале (медиана) снижался с 7,0 до 4,5 в течение 15 дней после первого введения тапентадола (р<0,05). У 22 (55%) пациентов показатель оценки боли улучшился более чем на 33%. В Италии проведено исследование эффективности тапентадола у 22 пациентов, которые испытывали сильную НБ после лечения таксанами и препаратами платины. До назначения тапентадола они не принимали опиоиды. Терапия препаратами первой линии (габапентиноиды, амитриптилин) была для пациентов неэффективной. Тапентадол применяли в течение 3 месяцев. Отмечено уменьшение интенсивности боли, оцененной по 10-балльной шкале: на 30% у 19 (86%) из 22, на 50% у 15 (68%) из 22 пациентов. Также достоверно снизилась выраженность невропатического компонента боли, по оценкам, с использованием опросника DN4 [39]. Также была оценена эффективность тапентадола у пациентов с умеренной и тяжелой НБ, вызванной метастазами в кости, и проходящих паллиативную 3D-лучевую терапию [40].
В исследование были включены 17 участников (13 мужчин, 4 женщины), средний возраст которых составил 67 (42–81) лет. Средняя доза тапентадола, введенная перед лучевой терапией, составила 100 мг/сут (100–300 мг).
Было констатировано, что паллиативная лучевая терапия в сочетании с тапентадолом приводит к уменьшению НБ и улучшению качества жизни пациентов с метастазами в кости. В обсервационном исследовании ретроспективно проанализировали 25 пациентов с множественной миеломой, страдавших от НБ и получавших лечение тапентадолом (начальная доза – 100 мг/сут конечная средняя доза – 213,6±94,1 мг/сут) [41]. Тапентадол был высокоэффективен, снизив средний балл оценки боли по DN4 с 4,68±2,43 на исходном уровне до 0,41±0,91 на 12-й неделе лечения. Одновременно достоверно (р=0,01) улучшились все показатели качества жизни по опроснику SF-36. Было сделано заключение: тапентадол можно считать опиоидом первого выбора онкологических больных данной категории, страдающих от боли с невропатическим компонентом. Было выполнено ретроспективное обсервационное исследование 36 онкогематологических пациентов, страдавших от ноцицептивной и НБ с интенсивностью в диапазоне от 5 до 10 баллов по нумерологической рейтинговой шкале. Тапентадол (ПД) назначался в дозировках от 200 до 260 мг/сут (243,5±105,6) после тщательного титрования. Препарат значимо снизил боль и улучшил качество сна у больных [42]. Превосходство тапентадола над классическими опиоидами у пациентов с онкологической НБ продемонстрировано в прямом сравнительном исследовании препаратов [43]. В нем участвовали пять групп онкологических больных. Пациентам с НБ назначали тапентадол (n =29), метадон (n=32), оксикодон (n=20), фентанил (n=26), гидроморфон (n=20). Оценка эффективности проводилась по вербальной рейтинговой шкале (от 0 до 3 баллов). Также оценивалась переносимость каждого опиоида. Среднее снижение интенсивности боли по вербальной рейтинговой шкале было больше в группе тапентадола, чем в группе оксикодона (p=0,0024), метадона, фентанила и гидроморфона. Частота отмены препарата в группе тапентадола была самой низкой из всех групп в исследовании. Авторы сделали следующее заключение: тапентадол превосходит оксикодон, фентанил и гидроморфон по эффективности лечения НБ при раке. Также они сочли, что в силу хорошей переносимости тапентадол является предпочтительным вариантом лечения НБ у онкологических больных, нуждающихся в быстром увеличении дозы при ее титровании или имеющих высокий риск нежелательных эффектов.
Сравнительные оценки переносимости тапентадола при его применении в клинической практике
Во многих заключениях о результатах приведенных выше исследований наряду с высоким уровнем эффективности констатировался и хороший уровень переносимости тапентадола. Одним из наиболее частых и наиболее распространенных нежелательных эффектов при приеме опиоидов является опиоид-индуцированный запор. Для уменьшения его выраженности пациенты на регулярной основе применяют слабительные средства. Сравнительные исследования переносимости тапентадола с классическими сильными опиоидами, в частности с оксикодоном, выполнялись с момента начала клинического применения препарата. В работе M. Etropolski et al. [41] переносимость тапентадола в дозах 50 и 75 мг сравнивалась с переносимостью оксикодона в дозе 10 мг. Влияние этих доз тапентадола и оксикодона на функцию кишечника и переносимость со стороны ЖКТ были оценены с использованием валидизированного дневника функции кишечника, позволяющего оценивать симптомы и выраженность запора, вызванного опиоидами. Установили, что при приеме тапентадола в дозе 50 и 75 мг подавляющее действие на число дефекаций ниже, чем у оксикодона в дозе 10 мг. Частота тошноты и рвоты также была значительно ниже (р<0,001).
В мета-анализ, проведенный M. Merker et al. [45], было включено 9 исследований с участием 7948 пациентов, из них 2810 получали оксикодон, 5138 – тапентадол в эквивалентных обезболивающих дозировках. Результаты включенных в обзор исследований демонстрировали меньшее число развития тошноты, рвоты, запора, головокружения и сонливости при применении тапентадола по сравнению с оксикодоном.
В Германии было выполнено сравнительное исследование воздействия тапентадола и других сильных опиоидов на моторику ЖКТ в условиях реальной клинической практики [46]. Исследование было ретроспективным когортным с использованием данных медицинского страхования пациентов, которым были назначены сильные опиоиды после предварительного лечения другими препаратами. Анализ проведен с даты первого назначения препаратов в 2015 г. в течение периода в 2 года.
Основным параметром для оценки негативного воздействия препаратов на кишечник был учет назначения пациенту слабительных средств. Оказалось, что значительно меньшему числу пациентов, получавших тапентадол ПД, в отличие от пациентов, получавших классические сильные опиоиды (оксикодон, морфин), назначались слабительные средства (20,3 против 37%; р<0,0001). Дозы слабительного также были значительно ниже в группе пациентов, получавших тапентадол (26,4 против 82,5 определенных суточных доз; р<0,0001). В последние годы для снижения риска развития опиоид-индуцированного запора широко применяется лекарственный препарат, содержащий оксикодон в комбинации с налоксоном.
В исследование R. Baron [47] были включены 258 пациентов c интенсивностью боли ≥6 баллов по 10-балльной шкале, у которых сравнивалось влияние тапентадола и оксикодона/налоксона на моторику кишечника. Титрование дозы препаратов проходило в течение 3 недель начиная с дозы тапентадола 50 мг 2 раза в сутки или оксикодона/налоксона 10 мг/5 мг 2 раза в сутки. После 21-дневного титрования максимальные лечебные дозы составили: тапентадол ПД – 250 мг 2 раза в сутки или оксикодон/налоксон ПД – 40 мг/20 мг 2 раза в сутки. Подобранные дозы препаратов принимали в течение 9 недель. При применении тапентадола ПД частота запоров и рвоты была ниже, чем при применении оксикодона/налоксона ПД (р≤0,045). Улучшения в индексе состояния здоровья по измерению EuroQol-5 и оценке качестве жизни были значительно выше при использовании тапентадола ПД по сравнению с оксикодоном/налоксоном ПД (р≤0,024). Были сделаны выводы: тапентадол ПД оказывал меньшее отрицательное влияние на функцию кишечника и больше улучшал показатели качества жизни пациентов по сравнению с оксикодоном/налоксоном ПД.
В работе I.V. Rivera [48] проведено сравнение способности вызывать опиоид-индуцированный запор между тапентадолом, оксикодоном/налоксоном, гидроморфоном, трансдермальной терапевтической системой (ТТС) с фентанилом и ТТС с бупренорфином. Из 180 пациентов (средний возраст – 61,5 год; 66,7% женщин, средняя продолжительность лечения – 3 года) 57,2% страдали от ноцицептивной боли, 33,9% от смешанной боли и 8,9% от НБ. Наиболее часто назначаемыми опиоидами были оксикодон/налоксон (44,4%) и тапентадол (37,8%). Профиль функции кишечника был более благоприятным при применении тапентадола и оксикодона/налоксона без существенных различий между ними.
Завершая обсуждение в данном разделе, нужно отметить, что в рамках оценки переносимости и безопасности опиоида, конечно, важна сравнительная оценка его аддиктивного (наркогенного) потенциала. Однако этот вопрос выходил за рамки данного обзора, но был ранее рассмотрен автором в двух предыдущих публикациях [49, 50].
Заключение
Обзор публикаций по применению тапентадола у онкологических пациентов подтверждает, что на этапе клинических исследований до регистрации и на протяжении всего времени использования после внедрения в клиническую практику препарат демонстрирует анальгетическую эффективность при болях онкологического генеза, аналогичную «золотым» стандартам сильных опиоидов – морфину и оксикодону. Большинство авторов отмечают преимущество в эффективности тапентадола у пациентов с невропатическим или смешанным вариантом боли перед другими опиоидами, объясняя это наличием у молекулы препарата не только активности в отношении мю-опиоидных рецепторов, но и механизма, позволяющего ингибировать обратный захват норадреналина в синапсах. Констатируя высокий уровень анальгетической активности, многие исследователи отмечают лучшую переносимость тапентадола по сравнению с оксикодоном и другими классическими сильными опиоидами. В частности, это относится и к чрезвычайно актуальной для пациентов проблеме опиоид-индуцированного запора и других проявлений нежелательных эффектов действия опиоидов на ЖКТ. По влиянию на моторику кишечника тапентадол действует аналогично комбинированному препарату, содержащему оксикодон и налоксон, а в ряде исследований демонстрирует его лучшую переносимость пациентами.



