Обоснование
Заболеваемость и смертность от пневмококковой инфекции остается высокой во всем мире [1, 2]. Возбудителем является грамположительная патогенная бактерия Streptococcus pneumoniae, на сегодняшний день известно более 90 серотипов возбудителя. Пневмококк может вызывать заболевания различной степени тяжести: от острого среднего отита (ОСО) до внебольничной пневмонии (ВП), в некоторых случаях требующих госпитализации, а также инвазивную пневмококковую инфекцию – менингит, бактериемическую пневмонию и сепсис. Некоторые группы населения имеют более высокие риски развития тяжелой инфекций (например, дети раннего возраста, иммунокомпрометированные и пожилые люди) [3–5].
Наиболее экономически эффективной мерой профилактики пневмококковой инфекции является вакцинация. В 1977 г. разработана первая полисахаридная вакцина против пневмококковой инфекции (ППВ). Однако, несмотря на широкий серотиповой состав, ППВ имеют ряд недостатков,в первую очередь низкую эффективность для детей раннего возраста. Поэтому с 2000 г. в медицинскую практику стали внедрять пневмококковые конъюгированные вакцины (ПКВ), которые в своем составе имеют капсульный полисахарид, конъюгированный с белкомносителем (дифтерийный или столбнячный анатоксины). ПКВ в отличие от ППВ вызывают Т-зависимый иммунный ответ и вырабатывают долговременную иммунную память, что при повторном введении вакцины приводит к значительному нарастанию титров антител. При частых ревакцинациях ППВ, наоборот, возникает риск гипореспонсивности. ПКВ применяют в мире в отношении детей с 2 месяцев жизни, в т.ч. недоношенных. Включение конъюгированной вакцины в национальные программы их иммунизации привело к формированию коллективного иммунитета и снижению заболеваемости у взрослых, а также к уменьшению распространенности антибиотикорезистентных серотипов S. pneumoniae [1, 6, 9]. В нашей стране профилактика пневмококковой инфекции с использованием ПКВ13 включена в Национальный календарь прививок с 2014 г.
Серотиповой состав ПКВ также расширяется: первая ПКВ содержала защиту против 7 основных серотипов S. pneumoniae, в настоящий момент в календаре прививок применяется ПКВ13 [7], в США зарегистрированы для взрослых ПКВ15 и ПКВ 20 [8].
В период пандемии коронавирусной инфекции в мире произошло снижение своевременной вакцинации детей, это привело, в частности, к росту заболеваемости пневмококковыми инфекциями. Стабильное эпидемиологическое благополучие обеспечивает высокий уровень своевременной вакцинации, поэтому Всемирная организация здравоохранения декларировала о необходимости формирования во всех странах «догоняющей иммунизации», одним из принципов которой является одновременное введение всех необходимых по возрасту вакцин.
Цель исследования: оценить реактогенность ПКВ при применении ее раздельно и в сочетании с вакцинами календаря прививок, а также оценить клинико-эпидемиологическую эффективность своевременности вакцинопрофилактики пневмококковой инфекции.
Методы
Проведено ретроспективное исследование. В анализ были включены 50 детей, привитых против пневмококковой инфекции с использованием ПКВ, в возрасте от 3 месяцев до 3 лет включительно (средний возраст – 10,07±3,73 года, соотношение полов примерно одинаковое – 23 мальчика и 27 девочек). По данным анамнеза, 12 (24%) детей были здоровыми, 76,0% имели различные заболевания, в т.ч. аллергические – 8 (16%), неврологические (резидуально-органическое поражение центральной нервной системы, гидроцефалия, детский церебральный паралич, симптоматическая эпилепсия, перинатальная энцефалопатия) – 5 (10%), ЛОР-патологию (хронический аденоидит, хронический тонзиллит) – 2 (4%), соматические (гипотрофия) – 2 (4%), бронхолегочную дисплазию – 1 (2%), сочетанную патологию (внутриутробные инфекции, поражение центральной нервной системы, аллергические проявления, частые заболевания) – 20 (40%).
Критерии включения: доношенные дети в возрасте от 3 месяцев до 3 лет, не имеющие иммуносупрессивных заболеваний и не получающие иммуносупрессивную терапию.
Вакцинация проводилась по графику, предусмотренному действующей версией Национального календаря профилактических прививок и актуальной инструкцией к препарату: дети до 11 месяцев включительно получили 2 вакцинации (до 6 месяцев включительно с интервалом не менее 8 недель, с 7 до 11 месяцев с интервалом не менее 4 недель), ревакцинацию не ранее 15 месяцев жизни, дети с 12 до 23 месяцев включительно получали 2 дозы вакцины с интервалом не менее 8 недель, дети с 24 месяцев жизни и старше прививались однократно.
Источником данных служила медицинская карта ребенка (форма 112/у).
Для оценки реактогенности вакцины анализировали данные о состоянии здоровья детей в течение 3 дней после вакцинации (опрос о состоянии здоровья ребенка медицинской сестрой по телефону), а в течение месяца после вакцинации учитывали развитие острых инфекций или обострение хронического заболевания. При наслоении интеркуррентного заболевания или обострении хронической патологии в течение 3 дней после иммунизации поствакцинальный период оценивали как осложненный.
Клиническая эффективность вакцинации оценивалась по сравнению с группой непривитых детей (родители которых отказывались от проведения вакцинации), проживавших в тех же условиях, по заболеваемости детей ОСО и ВП на протяжении 1–4 лет после вакцинации с расчетом доли детей, перенесших отит или пневмонию, от числа детей в группе непривитых и привитых.
Этическая экспертиза исследования не проводилась. На изучение медицинской документации получали согласие родителей или опекунов. Данные, полученные из медицинской документации для анализа, были обезличены использованием инициалов, возрастом в месяцах с отсутствием даты рождения.
Статистический анализ полученных результатов осуществлялся с применением пакета прикладных программ Microsoft Excel 2010 и Statistica 7.0 for Windows. Для оценки профилактической эффективности вакцинации использовался коэффициент эффективности (Е) и индекс эффективности (К), которые рассчитывались по формулам: Е=100*(b-a)/b(%) и K=b/a; где a – заболеваемость среди вакцинированных, b – заболеваемость среди невакцинированных. Показатель заболеваемости человек–время PtR (показатель плотности инцидентности) рассчитывался по формуле PtR=N/ ЧЧЛ*1000 (‰), где N – число новых случаев болезни за период наблюдения в группе, ЧЧЛ – число человеко-лет наблюдения.
Результаты
Оценка безопасности и реактогенности ПКВ
В целом дети получили 108 доз ПКВ, из них 60 в сочетании с другими вакцинами, 48 раздельно. У всех привитых поствакцинальный период протекал гладко, в 92,6% случаев бессимптомно: после первой вакцинации – у 90% привитых, после второй – у 91,4%, после 3 – у 100% (рис. 1). После 3 вакцинаций (108 доз) нормальные общие вакцинальные реакции зарегистрированы в 8 (7,4%) случаях, местные – в 5 (4,6%). Выраженность и частота нормальных вакцинальных реакций не зависели от состояния здоровья детей (в 6,3% у детей с фоновой патологией и 10,7% у здоровых). Отмечалась тенденция к большей частоте нормальных вакцинальных реакций при сочетанном введении препарата (в 10%), чем при раздельном (4,2%). Серьезных побочных эффектов после иммунизации ПКВ здоровых детей и с различными изменениями в состоянии здоровья зарегистрировано не было.
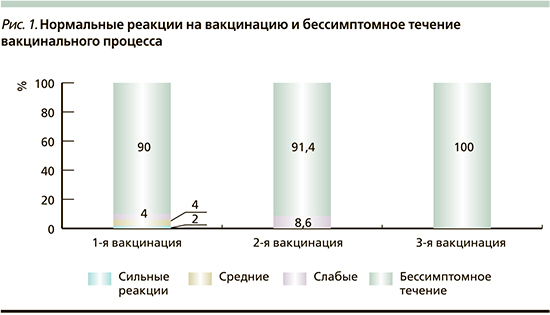
После 4-го дня вакцинации в течение месяца в 24,1% (от числа использованных 108 доз) возникли интеркуррентные инфекции или обострение хронических заболеваний с одинаковой частотой у детей здоровых (21,4%) и с фоновой патологией (26,3%). Отмечена выраженная тенденция к снижению частоты заболеваний с увеличением кратности вакцинации (34,0% после первой дозы, 20,6% после второй и 5,7% после третьей).
Таким образом, использование ПКВ c вакцинами календаря прививок не влияет на безопасность иммунизации. ПКВ является высокобезопасным препаратом для детей здоровых и с хронической патологией.
Клинико-эпидемиологическая эффективность вакцинации ПКВ
Профилактическая эффективность ПКВ определялась на основании сравнения показателей заболеваемости ОСО и ВП в группе привитых (n=50) и в группе сравнения (n=100). Различий по возрасту, сопутствовавшей заболеваемости и факторам риска развития пневмококковой инфекции между группами не выявлено. На протяжении всего периода наблюдения все участники исследования пребывали в одинаковых социально-бытовых условиях. Рассчитанные показатели заболеваемости пневмониями любой этиологии на 1000 детей в группе привитых были ниже в течение всего периода наблюдения (рис. 2). Показатель заболеваемости пневмониями с учетом человеколет (показатель плотности инцидентности) за весь период наблюдения в группе привитых составил 9,7 на 1000 человеко-лет (95% доверительный интервал [ДИ]: 9,1–10,3), в группе сравнения – 92,6 на 1000 человеко-лет (95% ДИ: 91,3–93,9). Индекс эффективности вакцинации в отношении пневмоний любой этиологии составил 9,5%, коэффициент эффективности – 89,5.
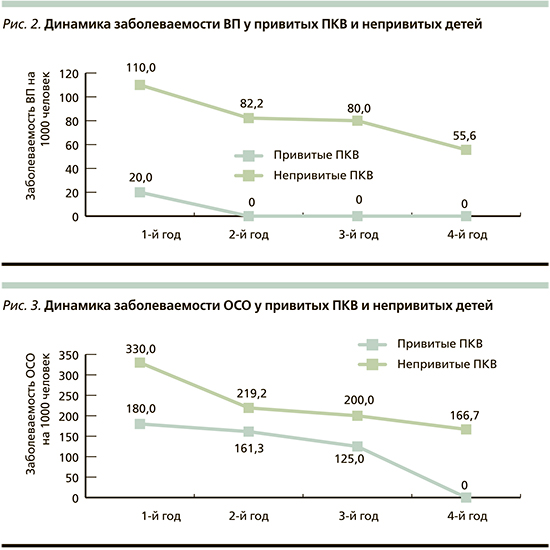
Анализ расчетных показателей заболеваемости отитами выявил в обеих группах снижение заболеваемости к 4-му году наблюдения, что может быть связано с повзрослением детей, однако этот показатель у непривитых был выше во все сроки (рис. 3). Показатель инцидентности в основной группе был в 1,8 раза меньше – 155,3 на 1000 человеко-лет (95% ДИ: 150,9–155,7), в контрольной группе – 263,9 на 1000 человеко-лет (95% ДИ: 261,7–266,1). Индекс и коэффициент эффективности вакцинации в отношении ОСО составили 1,8 и 44,3%.
Таким образом, эффективность вакцинации была более выраженной в отношении пневмоний по сравнению с отитами, что соответствует данным, полученным в разных странах мира [1].
Обсуждение
Установлено, что S. pneumoniae является основным возбудителем ВП и ОСО, в связи с чем выявленная тенденция снижения частоты возникновения данных заболеваний у привитых ПКВ детей служит прямым доказательством эффективности вакцинации, что подтверждается данными зарубежных исследователей [10, 11]. В патогенезе острых респираторных инфекций развитие бактериальных инфекций в большинстве случаев вторично по отношению к вирусному заболеванию. В условиях пандемии новой коронавирусной инфекции (НКИ, COVID-19) бактериальная коинфекция также преобладает у пациентов с COVID-19 (распространенность ко-/вторичной инфекции, связанной с COVID-19, достигает 45,0%). При этом S. pneumoniae является наиболее частым коинфицирующим возбудителем. НКИ в настоящее время считается основным фактором риска пневмококковой пневмонии и инвазивной пневмококковой инфекции. Вирус SARS-CoV-2 угнетает работу иммунной системы, что создает благоприятные условия для патогенных микроорганизмов, в т.ч. для пневмококка. Кроме того, факторы риска по тяжелому течению НКИ и пневмококковой инфекции в большинстве случаев совпадают. Таким образом, пневмококковая вакцинация во время пандемии COVID-19 стала как никогда актуальной [12, 13].
Заключение
ПКВ безопасна и малореактогенна для здоровых детей и с различной хронической патологией, общие и местные реакции развиваются редко, с одинаковой частотой в обеих группах. Вакцинация против пневмококковой инфекции эффективна (снижается частота ОСО и ВП у детей разного возраста) и должна проводиться как можно раньше в рамках Национального календаря профилактических прививок (с 2 месяцев), в случае нарушения графика – по схемам догоняющей вакцинации.


