Введение
Люминальный HER2- рак молочной железы (РМЖ) является наиболее распространенным подтипом РМЖ [1]. Этим обусловлен и бурный темп развития в лечении метастатического люминального HER2- РМЖ (HR+HER2-мРМЖ). Так, регулярно обновляемые рекомендации обсуждают такие сложные для переноса в клиническую практику определения, как «висцеральный криз» и «эндокринорезистентность» [2].
Преодоление эндокринорезистентности – это чрезвычайно актуальная проблема в лечении HR+HER2- мРМЖ. Так, появившиеся всего несколько лет назад CDK4/6-ингибиторы уже прочно вошли в клиническую практику. Результаты регистрационных исследований демонстрируют и увеличение выживаемости (как безцецидивной, так и общей), и управляемый профиль токсичности, позволяющий безопасно проводить лечение в амбулаторных условиях при адекватном мониторинге токсичности.
Наиболее активно изучаемыми за пределами CDK4/6-ингибиторов является группа ингибиторов сигнального пути PI3K/AKT/mTOR. Эверолимус, продемонстрировавший свою эффективность в исследовании BOLERO-2, долгое время в комбинации с эксеместаном занимал свое место в лечении HR+HER2- мРМЖ после прогрессирования на ингибиторах аромазаты и фулвестранте [3].
В 2019 г. ободрение FDA (Food and Drug Administration) для пациентов с HR+HER2- мРМЖ с наличием мутации в гене PIK3CA получил еще один ингибитор этого сигнального пути – алпелисиб.
Регистрационные исследования алпелисиба: SOLAR-1 и BYLieve
Исследование SOLAR-1 – это рандомизированное двойное слепое плацебо-контролируемое исследование III фазы, в которое были включены 572 пациента с HR+HER2- мРМЖ, имевшие прогрессирование на предшествовавшей терапии ингибиторами ароматазы [4]. До начала терапии и рандомизации в ткани опухоли у всех пациентов был определен статус мутации в гене PIK3CA, 341 пациент имел мутацию.
Пациенты были рандомизированы в соотношении 1:1, первая группа получала комбинацию алпелисиба 300 мг/сут и фулвестранта 500 мг дни 1,15, 29, далее – ежемесячно; вторая – комбинацию плацебо и фулвестранта в тех же дозах. Стратификация проводилась в зависимости от наличия висцеральных метастазов и терапии CDK4/6-ингибиторами в анамнезе. Обе группы были сопоставимыми по основным характеристикам. Первичной контрольной точкой исследования была выживаемость без прогрессирования (ВБП) в группе пациентов с наличием мутации PIK3CA, ключевой вторичной контрольной точкой – общая выживаемость (ОВ) в той же группе, к остальным вторичным контрольным точкам – ВБП в группе пациентов без мутации, ОВ в группе пациентов без мутации, частота объективных ответов и безопасность терапии.
Результаты исследования продемонстрировали эффективность комбинации в группе пациентов, имевших мутацию в гене PIK3CA. ВБП составила 11 месяцев в группе комбинации против 5,7 в контрольной группе (p<0,001). Частота объективных ответов составила 26,6 против 12,8% в пользу пациентов, получавших исследуемый препарат. Наличие стратификационных факторов (наличие висцеральных метастазов, использование CDK4/6-ингибиторов) не влияло на эффективность комбинации в исследуемой группе. Следует отдельно подчеркнуть, что статистически значимая разница не была продемонстрирована в группе пациентов без мутации. Таким образом, использование комбинации целесообразно только пациентами, у которых подтверждено наличие мутации в гене PIK3CA.
Результаты анализа ОВ были представлены на ESMO-2020 [5]. К сожалению, несмотря на численную разницу (39,3 против 31,4 месяца) в группе пациентов с мутацией, не было достигнуто статистически значимой разницы в обеих группах. Таким образом, увеличивая ВБП, алпелисиб не увеличивает ОВ, тем не менее остается зарегистрированной опцией терапии в комбинации с фулвестрантом пациентов с подтвержденной мутацией в гене PIK3CA, имевших прогрессирование на предшествовавшей эндокринотерапии.
Стоит отметить, что в исследовании SOLAR-1 лишь малая часть пациентов, а именно 35, получали в анамнезе лечение CDK4/6-ингибиторами. С целью установить эффективность алпелисиба после предшествовавшей терапии CDK4/6-ингибиторами было проведено открытое исследование II фазы BYLieve [6].
На ASCO-2020 были доложены результаты лечения в когорте А, куда были включены пациенты, имевшие прогрессирование на предшествовавшей комбинации ингибиторов ароматазы и CDK4/6-ингибиторов, получавшие в рамках исследования терапию комбинацией фулвестранта и алпелисиба. В данную когорту были включены 127 пациентов. После 6 месяцев терапии не имели признаков прогрессирования 50,4% пациентов (95% доверительный интервал [ДИ]: 41,2–59,6%, нижняя граница достоверности – 30%), таким образом был достигнут запланированный уровень достоверности. Это исследование подтвердило целесообразность использования алпелисиба после предшествовавшей терапии CDK4/6-ингибиторами.
Нежелательные явления (НЯ) алпелисиба: фокус на кожной токсичности
Помимо продемонстрированной эффективности в обоих исследованиях интересен и профиль токсичности препарата. Данные по токсичности, полученные в исследовании SOLAR-1, представлены в табл. 1.
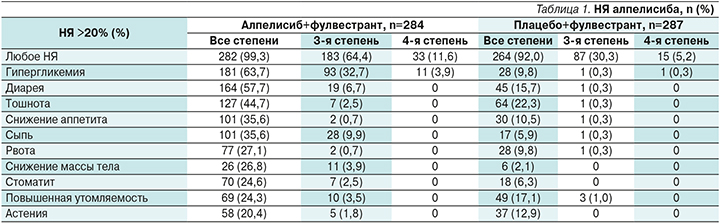
Как следует из данных, НЯ любой степени чаще наблюдались в исследуемой группе – 99,3 против 92,0%. НЯ 3–4-й степеней также чаще сопровождали терапию алпелисибом – 76 против 35,5%.
Наиболее специфичными и наиболее часто встречавшимися НЯ 3–4-й степеней были гипергликемия и сыпь. Мы не акцентируем в нашем обзоре внимание на диарее, поскольку рекомендации в данном случае соответствуют рекомендациям по лечению диареи, развившейся на фоне других лекарственных препаратов [7]. Рекомендации по гипергликемии также на сегодняшний день разработаны и опубликованы, в т.ч. в отечественных источниках [8].
Сыпь регистрируется у 40% пациентов, получающих лечение алпелисибом. У 3,2% пациентов сыпь стала причиной прекращения терапии в исследовании SOLAR-1. Наиболее частой локализацией поражения являются туловище (78%) и конечности (70%). Диффузная сыпь возникает в 13% случаев. Медиана времени до возникновения этого НЯ составляет 2 недели, а на разрешение уходит в среднем неделя [9]. Чаще всего сыпь представлена папулами и пятнами, возможно и наличие пустулезных элементов. Однако при обширном поражении кожных покровов возможно присоединение суперинфекции. Градация по степени тяжести и тактика лечения в зависимости от степени тяжести представлены в табл. 2.
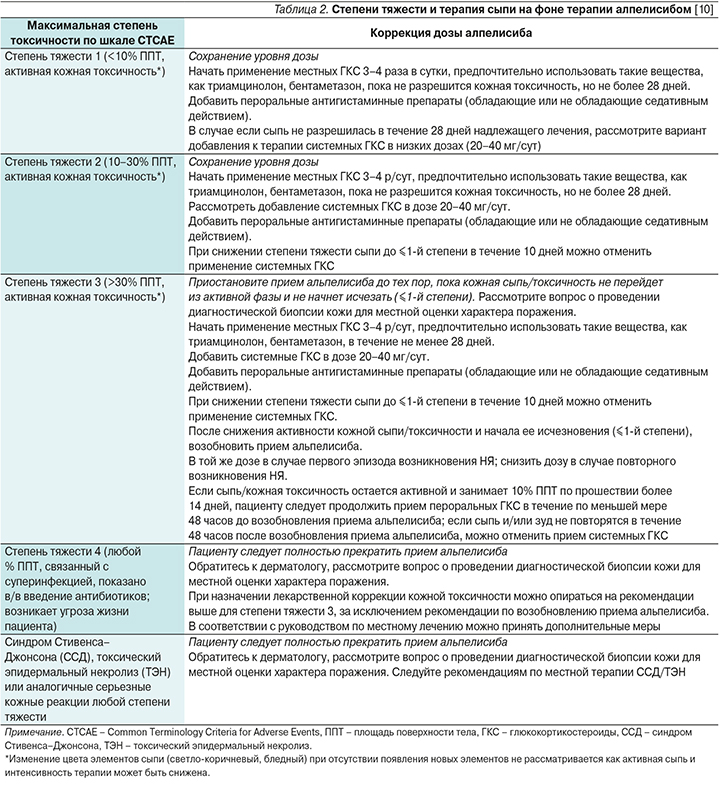
В случае если сыпь имеет пустулезные элементы, возможно назначение топических антибиотиков (клиндамицин 1–2%, гель или раствор эритромицина 1–2%, метронидазол 1%). Использование топических антибиотиков нежелательно при наличии зуда и нарушении целостности кожного покрова. В случае зуда возможно использование прамокаина 1%, доксепина 5% 2 раза в сутки. В отсутствие эффекта стоит рассмотреть возможность использования габапентина или прегабалина [11].
Кожная токсичность может значимо ограничивать функциональную активность пациентов и доставлять им значимый дискомфорт. Проанализировав токсичность терапии по данным исследования SOLAR-1, Hope Rugo и соавт. предложила использовать схему профилактики кожной сыпи [12]. Так, при использовании антигистаминных препаратов в исследуемой популяции частота сыпи снижалась с 53,9 до 26,7% (проанализированы все пациенты, включенные в исследование), а частота развития кожной токсичности 3-й степени снижалась с 20,1 до 11,6%. Таким образом, представляется целесообразным использование антигистаминной профилактики с целью предупреждения одного из самых частых НЯ. Рекомендовано использование препаратов, не имеющих седативного эффекта в течение 4 недель, предпочтительно назначение препаратов третьего поколения, таких как левоцитеризин (5 мг/сут), деслоратадин (5 мг/сут), фексофенадин (120–180 мг/сут) и рупатадин (10 мг/сут).
Клиническое наблюдение
Пациентке П. 1960 г.р. в 2017 г. установлен диагноз «рак правой молочной железы T2N0M0», гистологически – инфильтративный рак неспецифического типа, РЭ – 8, РП – 8, Her 2–1+, Ki-67 – 5%. С 10.2017 проводилось лечение: радикальная мастэктомия, 6 курсов адъювантной химиотерапии по схеме АС, с февраля по декабрь 2018 г. получала терапию летрозолом.
В декабре 2018 г. были выявлены метастазы в костях (до начала лечения сцинтиграфия не выполнялась). На первом этапе проведено 12 курсов паклитаксела в еженедельном режиме, лучевая терапия на поясничный отдел позвоночника, на правую половину таза, правый тазобедеренный сустав, верхнюю треть правого бедра (дозы неизвестны). Начата терапия препаратами, модифицирующими костный метаболизм. С февраля 2019 г. получала терапию комбинацией фазлодекса с рибоциклибом в стандартных дозах. В апреле 2020 г. выявлено прогрессирование за счет роста очагов в костях, появления очагов в печени. Начата терапия комбинацией эксеместана с эверолимусом. На этом фоне отмечена смешанная динамика, развитие лекарственного пульмонита не позволило продолжить терапию в прежнем режиме и оценить динамику. В августе 2020 г. выявлена мутация в гене PIK3CA.
С 18.09.2020 пациентка начала получать терапию в режиме эксеместан 25 мг/сут+алпелисиб 300 мг/сут. Эксеместан как препарат-партнер был выбран в связи с тем, что оценить его эффективность не представилось возможным в связи с развитием пульмонита, а терапия фазлодексом закончилась прогрессированием за полгода до этого.
Спустя 12 дней после начала терапии у пациентки была отмечена диффузная сыпь по всему телу (включая лицо и шею) пятнисто-папулезного характера, отдельные элементы сыпи сливались друг с другом. На этом фоне отмечено также повышение температуры до фебрильных значений (максимум – 38,3°С). Пациентка субъективно не отмечала наличия зуда. Фотографии сыпи представлены ниже (рис. 1, 2).
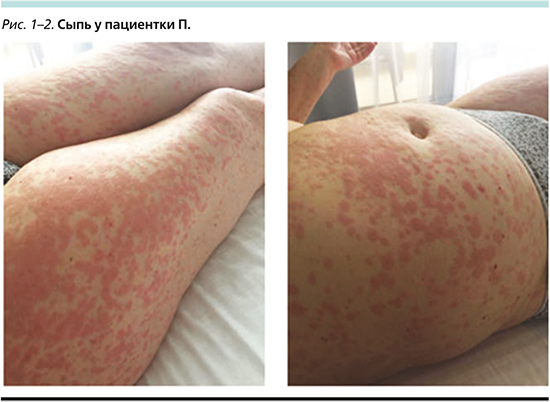
НЯ было расценено как кожная токсичность III степени. Терапия алпелисибом была немедленно остановлена. Начата терапия системными ГКС: метипред 40 мг/сут. Кроме того, пациентка самостоятельно начала прием цетиризина и местное применение диметендена. На этом фоне спустя 12 суток отметились явная положительная динамика, снижение температуры тела, разрешение сыпи до I степени. Начато постепенное снижение дозы системных ГКС. Алпелисиб возобновлен в дозе 250 мг спустя 14 суток после возникновения НЯ (1-й уровень снижения дозы). На этом фоне принимала фамотидин 40 мг/сут. За первые двое суток приема пациентка отметила возобновление покраснения и отечности элементов сыпи. Доза сразу же была снижена еще на 1 уровень – до 200 мг. На этом фоне сыпь полностью регрессировала к концу октября, ГКС были постепенно отменены, прекращен прием антигистаминных препаратов. Пациентка получала лечение в режиме эксеместан 25 мг/сут +алпелисиб 200 мг/сут.
Спустя 3 месяца терапии 26 января 2021 г. у пациентки было отмечено прогрессирование заболевания в виде появления новых очагов в костях, печени. С января 2021 г. по настоящее время (май 2021 г.) года пациентка получает терапию в режиме VinoCap.
В июне 2021 г. запланировано очередное обследование.
Обсуждение
В данном клиническом наблюдении представлено развитие кожной токсичности III степени на фоне терапии алпелисибом. Клинические проявления и сроки развития НЯ полностью согласовывались с описанными ранее в литературе: сыпь развилась в течение первых двух недель, преимущественно проявлялась пятнами и папулами. У данной пациентки также развилась фебрильная температура как ответ на генерализованный процесс: отмечалось вовлечение всех отделов тела, как наиболее часто поражаемых туловища и конечностей, так и лица и шеи.Поскольку лечение системными ГКС было начато незамедлительно после появления симптомов заболевания, удалось добиться уменьшения проявления токсичности спустя 2 недели после начала терапии. Более длительный срок разрешения НЯ по сравнению с данными литературы скорее всего связан с тяжестью НЯ.
К сожалению, данная терапия не была эффективной для пациентки. Тем не менее в данном клиническом примере можно наблюдать эффективность разработанной стратегии купирования кожной токсичности даже с учетом серьезности представленного НЯ.
Заключение
Баланс эффективности и переносимости терапии – наиболее важный аспект проведения противоопухолевого лечения пациентов с распространенным процессом. Современные препараты не только существенно расширяют возможности терапии, но и демонстрируют новые спектры токсичности. В настоящее время для большинства НЯ, в т.ч. и серьезных, разработаны алгоритмы действий, позволяющие безопасно проводить лечение и в полной мере реализовывать терапевтический потенциал лекарственных препаратов. Наиболее принципиальным здесь является тщательный мониторинг состояния пациента и своевременное назначение адекватной терапии.



