Введение
Рак шейки матки является наиболее часто встречаемой злокачественной опухолью женской половой системы в репродуктивном периоде. По данным всемирной статистической службы GLOBOCAN, рак шейки матки (РШМ) занимает 4-е место в структуре злокачественных новообразований, уступая раку молочной железы, колоректальному и раку легких [1]. Отмечается нарастание случаев РШМ среди женской популяции моложе 30 лет, часто имеющих уже «запущенные формы» [1, 2]. По прогнозам GLOBOCAN, к 2050 г. заболеваемость РШМ увеличится на 50%, достигнув более 1 млн новых случаев в год [1].
Лучевая терапия и хирургический метод лечения местнораспространенных форм РШМ наиболее эффективные и считаются стандартными [3]. Рецидив в зоне облучения возникает в диапазоне 10–40% случаев, отдаленные метастазы регистрируются у 35% пролеченных больных [4]. У 70% больных с III стадией, у 45% со II и у 24% с I стадиями имеет место диссеминация опухолевого процесса в первые 5 лет после радикального лечения [5].
Использование повышенных доз облучения приводит не только к уменьшению частоты прогрессирования, но и к лучевому повреждению тканей малого таза, что обусловливает высокий уровень развития постлучевых изменений и лимитирует дальнейшее продолжение терапии [3].
Последние десятилетия научные поиски посвящены разработке новых подходов к комбинированному лечению больных РШМ с включением различных вариантов неоадъювантной химиотерапии (НАХТ).
Вопрос о применении неоадъювантной химиотерапии в комбинированном или комплексном лечении больных РШМ активно изучается в зарубежных клиниках. Данные кокрановского мета-анализа, посвященного сравнению индукционной химиотерапии с последующей операцией и хирургического лечения на первом этапе при местнораспространенных формах РШМ, продемонстрировали увеличение показателей общей выживаемости на 23% (p=0,02), а безрецидивной выживаемости – на 25% (p=0,008) в группе индукционной химиотерапии. Кроме того, отмечается тенденция к снижению частоты развития рецидивов и метастазирования в исследуемой группе [6].
Отечественный опыт использования неоадъювантной химиотерапии в лечении местнораспространенных форм рака шейки матки невелик [7–9]. Несмотря на значительные темпы развития области применения НАХТ, ее роль остается спорной.
Цель исследования: оценить непосредственные результаты применения неоадъювантной дозоинтенсивной платиносодержащей химиотерапии больных местнораспространенной формой рака шейки матки IВ2–IIВ FIGO-стадии.
Дизайн. Одноцентровое нерандомизированное контролируемое проспективное исследование по изучению эффективности и токсичности неоадъювантной химиотерапии по схеме АР и ТР в дозоинтенсивном режиме с последующим хирургическим вмешательством в отношении больных местнораспространенным раком шейки матки начато в апреле 2016 г. в ФГБУ «НМИЦ онкологии им. Н.Н. Петрова» Минздрава России (протокол клинического испытания № 20). Статистическая обработка данных выполнена с применением «Statistica for Windows» v.10.0, StatSoft Inc. (США).
В исследование были включены 105 пациенток с морфологически подтвержденным диагнозом рака шейки матки IB2–IIB-стадии (FIGO)/T1B2–2BNX,0M0 (TNM), подписавших форму информированного согласия, возрастной категории от 19 до 70 лет, функциональный статус по ECOG 0; отсутствие выраженных отклонений в гематологических и биохимических показателях.
На этапе отбора больным проведено обследование, включившее стандартные клинико-лабораторные анализы крови, мочи, компьютерную томографию органов грудной клетки, брюшной полости и забрюшинного пространства, МРТ органов малого таза, цистоскопию и фиброколоноскопию, ЭКГ, ЭхоКГ.
Всем пациенткам проведено 3 цикла НАХТ в дозоинтенсивном режиме: 75 пациенткам по схеме АР (цисплатин 75 мг/м2, доксорубицин 35 мг/м2) и 30 пациенткам по схеме ТР (цисплатин 60 мг/м2, паклитаксел 60 мг/м2).
Внутривенное введение препаратов выполнялось каждые 10–14 суток.
До начала и по завершении НАХТ проведена видеофиксация состояния опухоли шейки матки с использованием специальной видеосистемы высокого разрешения VITOM (Karl Storz, Германия).
С целью оценки эффективности неоадъювантного лечения выполнена магнитно-резонансная томография (МРТ) органов малого таза, специализированная для шейки матки. Исследование проводилось дважды: до начала лечения и через 2 недели после окончания курса НАХТ.
Планирование дальнейшего этапа лечения проведено после оценки результатов контрольных исследований по критериям RECIST 1.1.
Степень повреждающего действия цитостатика (лечебный патоморфоз) определена морфологическим методом с оценкой общей структуры рака шейки матки, соотношения паренхимы, стромы и некроза, глубины и распространенности инвазии, состояния хирургического края резекции (параметриев, влагалища). За полный патоморфологический ответ принимали полное отсутствие инвазивной опухоли в образце.
Результаты
С июня 2016 г. в исследование были включены 105 первичных пациенток. Больные распределены на 2 группы в зависимости от схемы неоадъювантной химиотерапии: группу АР составили 75 пациенток (цисплатин в дозе 75 мг/м2, доксорубицин в дозе 35 мг/м2); группу ТР – 30 пациенток (цисплатин в дозе 60 мг/м2, паклитаксел в дозе 60 мг/м2).
Наиболее часто в обеих группах диагностировался умереннодифференцированный плоскоклеточный рак, превалировала IIB-стадия (62,7 и 63,3% соответственно). Клиническая характеристика больных представлена в табл. 1.
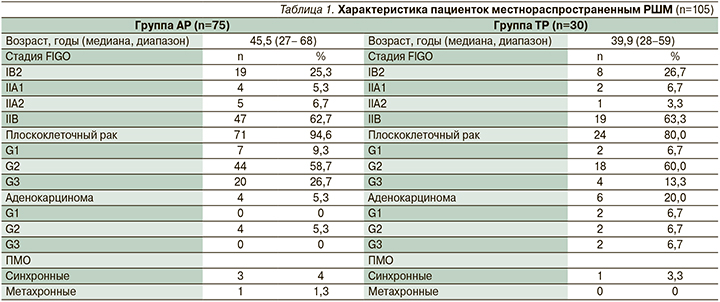
У 3 (4%) больных группы АР и у 1 (3,3%) группы ТР выявлены первично-множественные опухоли (ПМО). Спектр полинеоплазий представлен лимфогранулематозом, раком щитовидной железы, внутрипротоковой папиллярной муцинозной карциномой (IPMN) поджелудочной железы, пигментной меланомой. У 2 (2,7%) больных группы АР и у 1 (3,3%) группы ТР выявлены первично-множественные синхронные опухоли (пигментная меланома, рак щитовидной железы, IPMN поджелудочной железы), у 1 (1,3%) пациентки группы АР – первично-множественная метахронная опухоль (лимфогранулематозом).
Данные о профиле токсичности НАХТ представлены в табл. 2, оценка проведена согласно общей терминологии критериев для обозначения нежелательных явлений (CTCAE 4.03).
В группе АР оценена токсичность 226 курсов неоадъювантной химиотерапии 75 больных, в группе ТР оценена токсичность 90 курсов неоадъювантной химиотерапии 30 больных. Гематологиче-ская токсичность была умеренно выраженной и обратимой, в большинстве случаев проявлялась в виде анемии и лейкопении 1-й и 2-й степеней.
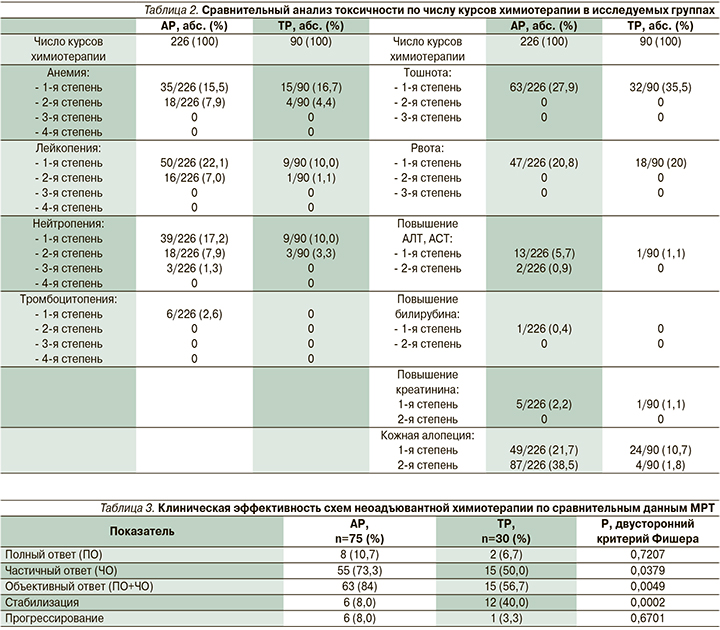
Негематологическая токсичность в обеих группах в основном была представлена эметогенной токсичностью 1-й степени, и кожной алопецией 1-й, 2-й степеней. Кожная алопеция встречалась достоверно чаще в группе АР (60,2 против 12,5% соответственно, р=0,0000), аллергических и неврологических реакций зафиксировано не было.
При анализе анемии достоверно чаще отклонения выявлялись в группе АР (23,4 против 21,1% соответственно, р=0,0005, критерий Манни–Уайта). Лейкопения также встречалась достоверно чаще в группе АР (29,1 против 11,1% соответственно, р=0,0005; критерий Манни–Уайта), нейтропения диагностировалась значимо чаще в группе АР (26,4 против 13,3% соответственно, р=0,0009; критерий Манни–Уайта).
На фоне проведенного лечения объективный ответ по критериям RECIST 1.1 для больных группы АР составил 84% (63 случая). Полный регресс опухоли был зафиксирован у 10,7% (8 больных), частичный ответ – у 73,3% (55 больных), стабилизация процесса отмечена в 8,0% (6 больных), прогрессирование заболевания выявлено в 8,0% (6 больных).
Среди пациенток, получивших курсы НАХТ по схеме ТР, клинический ответ распределился следующим образом: полный регресс опухоли был зафиксирован у 6,7% (2 больных), частичный – у 50% (15 больных), стабилизация процесса была отмечена в 40% (12 больных), прогрессирование заболевания выявлено в 3,3% (1 больной).
Объективный ответ в группе АР составил 84 против 56,7% в группе ТР (p=0,0049, двусторонний критерий Фишера, табл. 3).
В группе АР хирургическое лечение выполнено 66 (88%) больным из 75: оптимальные циторедуктивные операции в объеме радикальной экстирпации матки, двусторонней аднексэктомии, тазовой лимфаденэктомии (Piver III) выполнены 59 (78,7%) пациенткам; неоптимальные – 1 (1,3%), 6 (8,0%) пациенткам выполнена тазовая лимфодиссекция как этап хирургического стадирования с последующей химиолучевой терапией, 9 (12,0%) без хирургического стадирования была назначена химиолучевая терапия.
В группе ТР оптимальные циторедуктивные операции в объеме радикальной экстирпации матки, двусторонней аднексэктомии, тазовой лимфаденэктомии (Piver III) выполнены 23 (76,7%) пациенткам из 30, 1 (3,3%) пациентке выполнена тазовая лимфодиссекция как этап хирургического стадирования с последующей химиолучевой терапией, 6 (20%) без хирургического стадирования назначена химиолучевая терапия.
В группе АР 15 пациенткам был проведен радикальный курс сочетанной химиолучевой терапии (ХЛТ), в качестве радиосенсибилизатора использовался цисплатин в дозе 40 мг/м2 еженедельно: 6 (8,0%) пациенткам со стабилизацией опухолевого процесса, 6 (8,0%) больным с прогрессированием заболевания, а также 1 (1,3%) группы полного регресса, 2 (2,7%) пациенткам группы частичного регресса в связи с отказом от радикального хирургического лечения.
В группе ТР 7 пациенткам проведен радикальный курс сочетанной химиолучевой терапии: при стабилизации опухолевого процесса 4 (13,3%) больным, 1 (3,3%) пациентке с прогрессированием заболевания, а также 2 (6,7%) больным группы частичного регресса в связи с отказом от радикального хирургического лечения.
В исследуемых группах больных патоморфологический ответ был представлен как полный, неполный и отсутствие ответа. В группе АР неполный регресс опухоли на проведенное лечение встречался в 1,2 раза чаще, чем в группе ТР (78,8 против 62,5% соответственно, р=0,001). При сравнении частоты полных патоморфозов значимых различий в группах не выявлено (10,6 против 16,7% соответственно, р>0,05, табл. 4).
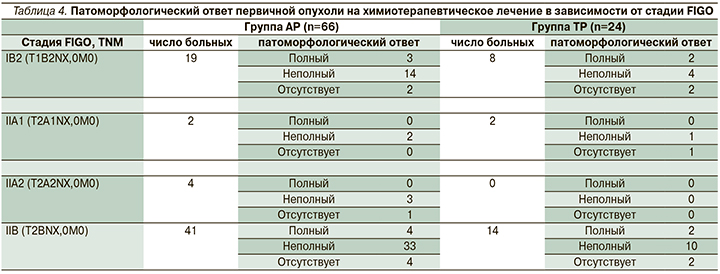
В группе больных, получивших НАХТ по схеме АР, патоморфологический ответ оценен для 66 больных (60 пациенток после радикальной гистерэктомии PIVER III, 6 – после тазовой лимфодиссекции) и составил 78,7%.
У 7 (10,6%) пациенток регресс опухоли подтвержден полным патоморфологическим ответом (ypCR).
В группе ТР патоморфологический ответ опухоли оценен для 24 больных (23 пациентки после радикальной гистерэктомии PIVER III, 1 пациентка после тазовой лимфодиссекции) составил 63,3%. У 4 (16,7%) пациенток регресс опухоли подтвердился полным патоморфологическим ответом.
У 7 (10,6%) больных группы АР морфологических признаков ответа первичной опухоли выявлено не было, у 9 пациенток не оценивался в связи с проведением радикального курса ХЛТ.
У 5 (20,8%) больных группы ТР патоморфологический ответ отсутствовал, у 6 пациенток не оценивался в связи с проведением курса конкурентной ХЛТ.
Для всех пациенток с прогрессированием и рецидивом заболевания после НАХТ по схеме АР морфологический тип опухоли был представлен низкодифференцированным плоскоклеточным раком. В группе ТР у пациентки с прогрессированием – умереннодифференцированным плоскоклеточным раком.
Пациенткам обеих групп проведено послеоперационное лечение: при негативных лимфатических узлах после радикального хирургического вмешательства проведен стандартный курс дистанционной конформной лучевой терапии, при выявлении метастатического поражения проводилась сочетанная ХЛТ.
Обсуждение
Выполненное нами исследование показало, что дозоуплотненная НАХТ является высокоэффективным методом лечения местнораспространенного РШМ, обеспечивая возможность выполнения радикального хирургического лечения больных первично неоперабельной опухолью шейки матки без увеличения частоты и выраженности интра- и послеоперационных осложнений.
Выбор схемы химиотерапии на сегодняшний день осуществляется эмпирически, данные о чувствительности и резистентности опухоли к лечению зачастую противоречивы.
Наиболее активным препаратом считается цисплатин с частотой клинического ответа не менее 20% при РШМ [6, 10]. Высокой эффективностью (от 17%, в ряде случаев достигая 40–50%) при лечении пациенток с местнораспространенным РШМ обладают противоопухолевые препараты из группы таксанов (паклитаксел), значимые результаты продемонстрированы при их совместном применении с цисплатином [11, 12].
Частота ответа рака шейки матки при использовании доксорубицина, по данным GOG, составляет 20%. Наиболее серьезным побочным эффектом является кардиотоксичность [13]. В нашем исследовании данный профиль токсичности не был отмечен.
Важным аспектом считается также интенсивность введения и доза препарата. В 1990 г. J.E. Sardi et al. первыми предложили новый, «быстрый», режим проведения НАХТ больным РШМ, позволивший получить в сжатые сроки максимальный терапевтический ответ при сравнительно неизменившимся профиле и уровне токсичности [14].
При сопоставлении данных с литературными при анализе необходимого числа курсов неоадъювантной химиотерапии, проведение 3 курсов химиотерапии оптимально для достижения клинического регресса опухоли при приемлемом профиле токсичности. Токсичность оценивалась по числу курсов химиотерапии. В большинстве случаев токсичность была умеренной, обратимой и проявлялась в виде 1-й и 2-й степеней, что коррелирует с данными литературы [15, 16]. Кумулятивной токсичности выявлено не было.
В обеих группах отмечена нулевая медиана, что означает, что более половины проведенных курсов химиотерапии не сопровождались токсическими явлениями.
Объективный ответ на неоадъювантную химиотерапию больных группы АР составил 56,7–84% в зависимости от режима химиотерапии. В исследовании H. Robova [17] данный показатель составил 78,8%, в то время как D.C. Park et al. продемонстрировали 90,7% [18, 19].
Частота радикального хирургического вмешательства варьировалаcь от 76,7–78,7% соответственно для исследуемых групп. Полный патоморфологический регресс выявлен в 10,6–16,7%.
Степень патоморфологического регресса опухоли после предоперационной химиотерапии, по данным литературы, может служить важным прогностическим фактором безрецидивной и общей выживаемости больных, однако в нашем исследования данной корреляции выявить не удалось, вероятно в виду малой событийности.
Заключение
Рак шейки матки высокоагрессивная злокачественная опухоль женской половой системы, занимающая первое место в репродуктивном возрасте. Это второй наиболее часто диагностируемый рак и третья причина смерти среди женщин, страдающих онкологическими заболеваниями [1, 2].
На текущий момент тактика ведения пациенток с местнораспространенным РШМ заключается в проведении радикального курса ХЛТ [3, 11]. Несмотря на доказанную эффективность, результаты лечения остаются неудовлетворительными, более 25% женщин умирают от прогрессирования заболевания на первом году после лечения, рецидив в зоне облучения возникает в 10–40% случаев, отдаленные метастазы регистрируются в 35% [5, 19].
Долгое время РШМ считался химиорезистентной опухолью. С конца 1980-х гг. основные исследования посвящены изучению роли различных вариантов неоадъювантной химиотерапии в комбинированном и комплексном лечении местнораспространенного рака шейки матки.
Результаты исследования свидетельствуют о клинической значимости дозоинтенсивной неоадъювантной химиотерапии в лечении РШМ, расширяют лечебные перспективы данной патологии.
Результаты работы могут служить основанием для планирования рандомизированного проспективного исследования по изучению эффективности и токсичности комбинированной платиносодержащей неоадъювантной химиотерапии больных местнораспространенным раком шейки матки для получения данных со значимым доказательным уровнем для последующей индивидуализации лечения.