Введение
Бронхиальная астма (БА) остается одним из наиболее распространенных хронических заболеваний органов дыхания, вовлекая в свою орбиту более 300 млн больных во всем мире [1], а к основным целям лечения БА относятся достижение контроля над болезнью, уменьшение числа ее повторных обострений и сведение к минимуму нежелательных лекарственных реакций [2]. При этом очевидная гетерогенность БА (в частности, случаи заболевания, связанные с физическими усилиями или инфекцией, протекающие на фоне ожирения, патологии верхних дыхательных путей или желудочно-кишечного тракта с частыми обострениями, эозинофилией периферической крови и т.д.) [3], дополненная данными сложных исследований клеточных и биохимических маркеров образцов индуцированной мокроты и биоптатов легочной ткани [4, 5], аргументирует реализацию концепции фенотип-/эндотип-специфической или пациент-ориентированной терапии [6]. Однако на практике БА, подобно многим другим распространенным заболеваниям человека, продолжает рассматриваться как достаточно однородное патологическое состояние, диагностические критерии которого не претерпели существенных изменений за последнее столетие, в связи с чем к ее лечению в подавляющем большинстве случаев сохраняется эмпирический подход [7]. А основу эмпирического подхода составляют ингаляционные глюкокортикостероиды (ИГКС), как правило, в комбинации с длительнодействующими β2-адреноагонистами (ДДБА) – ИГКС/ДДБА [8], применение которых большинством больных с симптоматическим течением БА может быть охарактеризовано принципом «One size fits all» («Один размер подходит всем»). Учитывая же тот факт, что данная группа лекарственных препаратов со временем становится все более представительной, перед практикующим врачом зачастую встает непростой вопрос о выборе «лучшего из возможного».
Доказательства эффективности и безопасности
Рандомизированные клинические исследования
Всем сторонам лечебного процесса – регулирующим организациям, страховым компаниям, пациентам, врачам – необходима надежная и достоверная информация о преимуществах, аспектах безопасности и экономической эффективности лекарственных средств, предполагающая поиск доказательств и использование их для принятия клинического решения [9].
А «несущей конструкцией» медицины, основанной на доказательствах, является иерархическая классификация, где систематические обзоры, мета-анализы и рандомизированные клинические исследования (РКИ) обычно рассматриваются как самый высокий уровень клинических доказательств [10]. РКИ располагаются на самом высоком уровне принятой иерархии, поскольку предполагается, что, будучи беспристрастными, они характеризуются минимальным риском систематических ошибок [11]. Тем не менее обычно демонстрируемые в почти идеальных условиях двойных слепых РКИ эффективность и безопасность изучаемых лекарственных препаратов по сравнению с активным контролем или плацебо не всегда отражают реальную клиническую практику [12].
Как известно, РКИ проводятся в тщательно отобранных высокоселективных популяциях больных, отвечающих строгому перечню критериев включения/невключения в исследование и потому лишь в малой степени соответствующих больным, встречающимся в повседневной клинической практике. Так, в частности, РКИ, проводимые среди больных с заболеваниями органов дыхания, характеризуются высокой внутренней валидностью, но часто представляют менее 5% от общего числа больных соответствующей нозологией, получающих лечение в плановом порядке. Например, в ходе одного из исследований показано, что из 334 больных БА, последовательно посетивших кабинет врача, только 11 (3,3%) могли быть включенными в традиционные РКИ [13]. Впрочем, в этом нет ничего удивительного, поскольку обязательное следование типичным критериям включения – конкретные параметры функции внешнего дыхания, их воспроизводимость, обратимость бронхиальной обструкции, отсутствие серьезных сопутствующих заболеваний, невключение курильщиков, указание на историю лечения ИГКС, определенная выраженность симптомов БА и др. – неминуемо приводит к «смещению» выборки, а именно к исключению пациентов как с самым тяжелым, так и с легким, хорошо контролируемым течением заболевания [14]. Следует принимать во внимание и драматические различия в комплаентности участвующих в РКИ пациентов, которая поощряется и регулярно контролируется врачом-исследователем при зачастую невысокой приверженности больных БА врачебным предписаниям, когда они получают лечение в «real-life» [15, 16].
Указанные выше ограничения не исчерпывают перечня всех «слабых сторон» РКИ. Стремление уменьшить изменчивость между сравниваемыми группами, ограничить влияние тех или иных переменных факторов (конфаундеров) на ожидаемый результат, застраховать себя в получении анонсируемой цели исследования (например, доказательства сопоставимой клинической эффективности изучаемого лекарственного средства и препарата сравнения) вольно или невольно приводит к ограничению размера выборки пациентов. А это в свою очередь делает обнаружение редких нежелательных лекарственных реакций менее вероятным.
Последнее можно проиллюстрировать, обратившись, в частности, к истории изучения и клинического применения небарбитуратного снотворного/седативного препарата талидомид, зарегистрированного в Европе в конце 1950-х гг. и быстро ставшего одним из самых продаваемых лекарственных средств в 46 странах мира. С учетом его эффективности и безопасности, основанных на результатах РКИ, талидомид вскоре стал широко использоваться и беременными в качестве антиэметика [17]. Но уже в конце 1961 г. на основании многочисленных сообщений (более 10 тыс.) о развитии врожденных уродств [18] дальнейшее применение талидомида было категорически запрещено [19]. Причины этой трагедии очевидны: это и отсутствие адекватных доклинических исследований по оценке тератогенности препарата, и необоснованное расширение круга показаний к его применению, в т.ч. беременными, и отсутствие исследований по оценке эффективности и безопасности талидомида в рамках реальной клинической практики и, конечно, ограниченное число пациентов, включенных в регистрационные РКИ.
Итак, несмотря на то что результаты двойных слепых РКИ составляют основу нормативной базы по утверждению новых методов лечения и их включению в клинические рекомендации, необходима дополнительная информация о профиле «польза/риск» исследуемых лекарственных средств в реальной практике.
Прагматичные рандомизированные клинические исследования
В отличие от стандартных РКИ прагматичные рандомизированные клинические исследования (пРКИ) позволяют оценивать клиническую эффективность лекарственного средства или вмешательства в представительной, специально не отбираемой популяции, куда в выборку оказываются включенными и пациенты с сопутствующими заболеваниями (рис. 1). При этом за больными осуществляется рутинный уход, не предполагающий дополнительных визитов к врачу, получаемая ими в течение длительного времени терапия не маскируется, а попыток влиять на приверженность врачебным рекомендация (комплаенс) не предпринимается [20].
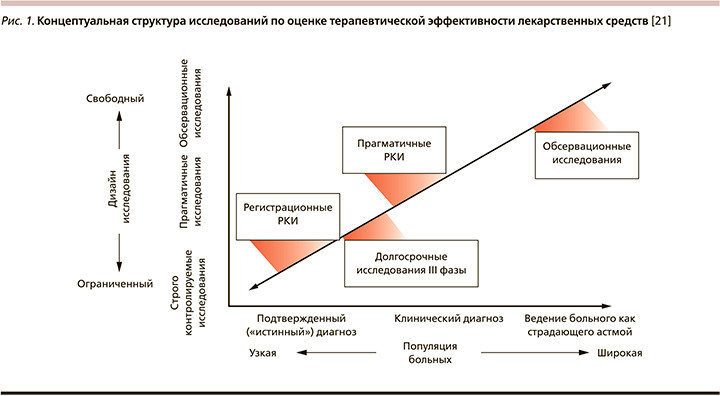
Прагматичные РКИ представляют собой большие по мощности выборки проспективных клинических исследований, в которых пациенты рандомизируются по двум или большему числу терапевтических вмешательств, а затем их ведение осуществляется в соответствии с повседневной врачебной практикой. Таким образом, пРКИ преодолевают разрыв между РКИ и нерандомизированными наблюдательными исследованиями, позволяя демонстрировать максимально эффективные стратегии лечения, актуальные для абсолютного большинства пациентов [22]. Однако пРКИ требуют охвата очень большого числа больных, что объясняет дороговизну и трудоемкость подобных исследований, а одним из их серьезных ограничений является вероятность «переключения» с одной лечебной стратегии на другую, что до некоторой степени способно обесценить рандомизацию [20]. Впрочем, несмотря на это, за последние годы наблюдается существенный рост числа публикаций, освещающих результаты пРКИ по целому ряду направлений терапии – от 43 в 1990 г. до 252 в 2010-м, хотя они по-прежнему составляют менее 1% от общего числа всех РКИ [22].
Salford Lung Study
В этом контексте Salford Lung Study (SLS), являющееся уникальным примером проведения прагматичных исследований до регистрации нового лекарственного средства в терапии больных БА и хронической обстуктивной болезнью легких (ХОБЛ), представляло собой 12-месячное открытое пРКИ III фазы по оценке эффективности и безопасности нового комбинированного препарата, содержащего современный ИГКС флутиказона фуроат (ФФ) и ДДБА вилантерол (ВИ) – 100 или 200 мкг/25 мкг, назначаемого 1 раз в день в форме многодозового порошкового ингалятора [23].
К участию в исследовании врачами общей практики приглашались больные БА, зарегистрированные в соответствующих базах данных всех 66 пунктов первичной медицинской помощи г. Солфорда (Salford)* и его окрестностей. Критериями включения больных БА в исследование стали: а) возраст ≥18 лет; б) симптоматическое течение БА, диагностированной врачом общей практики; в) регулярная поддерживающая терапия ИГКС или ИГКС/ДДБА; г) наличие симптомов БА в течение недели, предшествовавшей визиту 2.
Перечень критериев невключения был минимальным: а) анамнестические указания на недавний жизнеугрожающий астматический приступ; б) сопутствующая ХОБЛ или любое другое клинически значимое заболевание, способное поставить под угрозу безопасность пациента.
Дизайн SLS представлен на рис. 2.
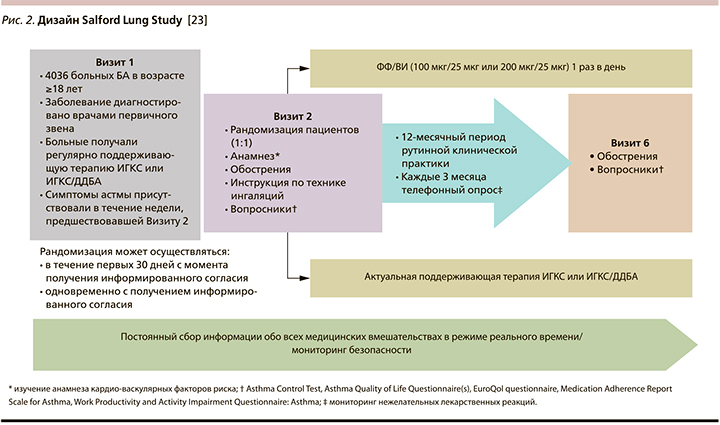
Уникальность SLS объясняется рядом инфраструктурных и клинических факторов. Это и относительно статичное, не подверженное существенным миграционным процессам население Солфорда, обслуживаемое одним госпиталем (Королевский госпиталь Солфорда), и использование для учета всех медицинских событий в процессе исследования единой электронной медицинской карты, фиксирующей информацию на уровне как пунктов первичной медицинской помощи, так и стационара (помимо этого использовались и дополнительные информационные каналы, позволившие судить об обращениях за медицинской помощью за пределами Солфорда, а также о случаях смерти) и участие в исследовании всех 55 городских аптек, что давало возможность надежно контролировать отпуск любого лекарственного препарата каждому пациенту.
Для оценки актуального уровня контроля БА использовался валидированный вопросник Asthma Control Test (АСТ) [24], заполнявшийся пациентами в начале и на 52-й неделе исследования в электронном виде, а на 12-й, 24 и 40-й неделях – по телефону [25], что позволяло, минимизируя число визитов к врачу, приблизиться к реальной клинической практике. Трактовка результатов опроса с использованием АСТ была традиционной: ≥20 баллов – «хорошо контролируемая», 16–19 – «частично контролируемая» и ≤15 баллов – «неконтролируемая» БА, а клинически значимой признавалась динамика суммарных значений АСТ ≥3 баллов [26].
В анализ первичной эффективности в общей сложности были включены 3026 больных БА с исходными значениями суммарного балла по АСТ <20, практически поровну распределенных между сравниваемыми группами: 1512 больных, принимавших ФФ/ВИ, и 1514 принимавших другие ИГКС или ИГКС/ДДБА («обычная терапия») [27].
Полученные результаты свидетельствовали, что уже на 24-й неделе исследования среди пациентов, принимавших ФФ/ВИ, число «ответивших» на лечение (АСТ ≥20 баллов и/или его увеличение на ≥3 баллов от исходных значений) оказалось достоверно бóльшим по сравнению с группой пациентов, принимавших «обычную терапию» (см. таблицу).
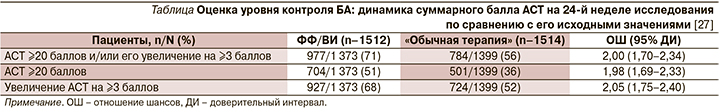
Примечательно, что выявленное на 24-й неделе превосходство в эффективности ФФ/ВИ над «обычной терапией» удалось продемонстрировать и на 52-й неделе исследования (рис. 3).
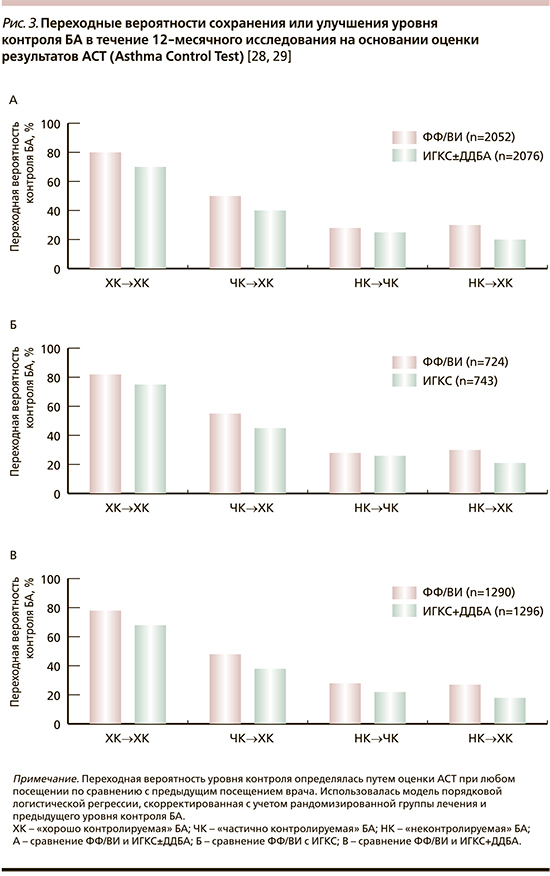
Важно также отметить, что результаты SLS свидетельствовали не только о том, что применение ФФ/ВИ обеспечивает лучший контроль БА по сравнению с продолжающейся «обычной терапией» (ИГКС±ДДБА) больных с симптоматическим течением заболевания, но и приводит к последовательному улучшению суррогатных параметров качества жизни, оцененных с помощью формализованных вопросников – Asthma Quality of Life Questionnaire(s) [29, 30], EuroQol questionnaire [31], Work Productivity and Activity Impairment Questionnaire: Asthma [32, 33] – независимо от исходного уровня контроля БА [28].
Обсуждение
Как уже говорилось выше, рекомендации по выбору оптимальных вариантов лечения того или иного заболевания, в т.ч. и БА, традиционно основываются на результатах двойных слепых РКИ [13, 33]. Однако отвечающие существующим требованиям регистрации новых лекарственных средств РКИ лишь в малой степени соответствуют реальной клинической практике.
А множественность критериев включения и невключения приводит к тому, что из общего числа больных БА лишь около 5% потенциально могут принимать участие в том или ином РКИ [13]. Кроме того, содержание отдельных РКИ представляет собой анализ ограниченных данных (например, оценка динамики объема форсированного выдоха за 1-ю секунду), немалая часть из них характеризуется небольшой продолжительностью и ограниченной мощностью выборки. Здесь же следует указать еще на одну причину разрыва между результатами реальной клинической практики и РКИ. Участвующие в последних больные, находясь под пристальным наблюдением врача-исследователя, чаще демонстрируют и более правильную технику ингаляционного маневра, и большую комплаентность, нередко достигающую 90% [23]. К сожалению, и наблюдательные исследования не могут предоставить достаточно объективную информацию в силу отсутствия подходящих групп сравнения и малого числа пациентов в ходе проспективного наблюдения [34]. Отсюда становится очевидным, что ни РКИ, ни наблюдательные исследования не могут в полной степени оценить истинное влияние и ценность той или иной лечебной стратегии для больных БА.
В преодолении объективно существующих ограничений РКИ и наблюдательных исследований особое место занимают пРКИ, в которых наблюдение и мониторинг пациентов сведены к минимуму. К числу подобных исследований относится и SLS – первое прагматичное рандомизированное исследование III фазы, начатое в то время, когда исследуемый препарат для лечения БА еще не был зарегистрирован.
Заключение
Продемонстрированное в ходе SLS терапевтическое превосходства ФФ/ВИ над «обычной терапией» в достижении контроля БА, объясняемое особенностями клинической фармакологии новой комбинации ИГКС+ДДБА, в т.ч. позволяющими принимать препарат 1 раз в сутки [27], а также привлекательная характеристика интуитивного многодозового порошкового ингалятора [36, 37] приближают к истинному пониманию профиля «польза/вред» ФФ/ВИ для существенно более широкой популяции больных.


