Введение
Инфекция Helicobacter pylori вызывает хроническое, прогрессирующее поражение слизистой оболочки желудка и играет ключевую роль в развитии таких заболеваний, как гастрит, язвенная болезнь 12-перстной кишки и желудка, аденокарцинома желудка и MALT-лимфома [1–4]. Исходы хеликобактерной инфекции зависят как от характеристик самого возбудителя, так и от иммуногенетики инфицированного человека.
С высокими уровнями доказательности и согласованности экспертами последних соглашений (Киото-2015 и V Маастрихт-2016) было принято важное положение, согласно которому H. pylori-ассоциированный гастрит является инфекционным заболеванием даже в отсутствие симптомов у пациента и вне зависимости от осложнений [3, 5]. Международное агентство по изучению рака (International Agency for Research on Cancer – IARC) классифицировало H. pylori как канцероген 1-го класса еще в 1994 г., отводя этой инфекции роль важного триггера в процессе канцерогенеза дистального рака желудка [6]. Экспертами киотского консенсуса особый акцент сделан на времени поиска H. pyloriассоциированного гастрита, зависящего от уровня распространенности инфекции в популяции и частоты развития рака желудка в зависимости от возраста [5]. Доказано, что эрадикация H. pylori сдерживает прогрессирование поражения слизистой оболочки, уменьшает резервуары инфекции и частично или полностью предотвращает H. рylori-ассоциированные заболевания [5]. Максимум пользы от эрадикации для пациента можно извлечь, если она проведена до появления таких предраковых изменений слизистой оболочки, как атрофия, кишечная метаплазия и дисплазия.
К основным компонентам схем эрадикационной терапии инфекции H. pylori относятся антибактериальные препараты. Эффективность лечения напрямую зависит от чувствительности микроорганизма к ним [7]. По данным российских исследований, выявлен низкий уровень резистентности Н. pylori к кларитромицину (менее 15%) и метронидазолу в большинстве регионов России [8–11]. Кроме популяционной резистентности к антибактериальным средствам на эффективность терапии влияет и ряд других факторов: приверженность пациентов лечению (комплаентность), качество применяемых ингибиторов протонной помпы (ИПП), язвенный или неязвенный характер повреждения слизистой оболочки желудка и предшествующий опыт использования антибактериальных препаратов конкретным больным [12]. Имеют значение и особенности лекарственного метаболизма ИПП (например, преобладание в популяции «быстрых» или «медленных» метаболизаторов). Есть данные, согласно которым в европеоидных популяциях европейских стран и России преобладают «быстрые» метаболизаторы ИПП, поэтому предпочтительными препаратами в схемах эрадикационной терапии обозначены рабепразол и эзомепразол [3, 12].
Согласно положениям последнего Маастрихтского консенсуса и рекомендациям Российской гастроэнтерологической ассоциации (РГА), к рекомендуемым мерам оптимизации эрадикационной терапии относят увеличение продолжительности лечения, предпочтение в схемах эрадикации эзомепразола и рабепразола, назначение двойных доз ИПП, добавление в схемы пробиотика, включение в схемы ребамипида, повышение приверженности пациентов лечению [3, 12]. Одной из мер, повышающих эффективность эрадикационной терапии, служит добавление препаратов висмута к классической тройной терапии (ИПП+кларитромицин+амоксицилл ин) [3, 12, 13, 21].
Цель исследования: изучение и сравнение эффективности двух схем эрадикационной терапии H. pylori-ассоциированных заболеваний (язвенная болезнь, гастрит): классической тройной (эзомепразол [Эманера] 40 мг 2 раза, кларитромицин: [Фромилид] 500 мг 2 раза, амоксициллин [Флемоксин] 1000 мг 2 раза), и классической тройной с добавлением висмута трикалия дицитрата (ВТД) (Улькавис 240 мг 2 раза).
Методы
Исследование выполнено на базе клиники НИИ терапии и профилактической медицины – филиала ИЦИГ СО РАН. Критерии включения в исследование: наличие у пациента инфекции H. pylori и показания к проведению эрадикационной терапии. Критерии исключения: возраст моложе 18 лет и старше 80 лет, наличие аллергии к антибактериальным препаратам (амоксициллин, кларитромицин), беременность, использование антибиотиков в течение 4 недель перед исследованием, проведение эрадикационной терапии в анамнезе и наличие тяжелых сопутствующих заболеваний.
В исследование были включены 138 пациентов с кислотозависимыми заболеваниями в возрасте от 19 до 78 лет (средний возраст – 50,1±13,6 года), преимущественно женщины (61,1%). Диагностика инфекции H. pylori проведена с использованием быстрого уреазного теста (Хелпил-тест, СанктПетербург) во время выполнения фиброгастродуоденоскопии (ФГДС).
Перед включением в исследование пациенты заполняли анкету (гастроэнтерологические жалобы, курение, потребление алкоголя, наличие профессиональных вредностей), составлялась родословная, фиксировались антропометрические показатели (рост, вес, индекс массы тела, окружность талии), проводилось общеклиническое обследование.
Случайным образом (по мере поступления в отделение) были сформированы 2 группы. Пациентам первой группы назначалась классическая тройная схема: эзомепразол (Эманера) 40 мг 2 раза в день, амоксициллин (Флемоксин) 1000 мг 2 раза в день, кларитромицин (Фромилид) 500 мг 2 ра- за в день, а пациентам второй группы – аналогичная тройная схема с добавлением ВТД (Улькавис) 240 мг 2 раза в день. Продолжительность лечения была одинаковой в обеих группах (10 дней). Во время терапии пациенты вели дневник с целью контроля приверженности лечению и его переносимости. Контроль за эрадикационной терапией проводился через 8 недель после окончания курса лечения с использованием уреазного дыхательного теста с 13С-мочевиной, иммуноферментного анализа антигенов Н. рylori в кале или быстрого уреазного теста при проведении ФГДС (если требовался контроль заживления дефекта, например, при язвенной болезни желудка).
Статистическая обработка результатов исследования проведена с применением программы SPSS (версия 11.0). Описательный анализ числовых характеристик при нормальном распределении признаков включал средние значения, стандартное отклонение, а при ненормальном распределении показателя полученные результаты были представлены в виде медианы и межквартильного размаха. Полученные данные в таблицах и тексте представлены как абсолютные и относительные величины (n, %), а также как (М±σ), где М – среднее арифметическое значение, σ – стандартное отклонение; и Me (25%; 75%), где Me – медиана, 25%, 75% – 1-й и 3-й квартилей. Достоверность различий для средних величин оценивали по критериям Фишера (F) и Стьюдента (t). Критерием статистической достоверности был уровень p< 0,05.
Исследование было одобрено локальным этическим комитетом НИИТПМ – филиал ИЦиГ СО РАН (протокол № 2 от 10.01.2017). Все пациенты подписали информированное согласие на участие в исследовании.
Результаты исседования
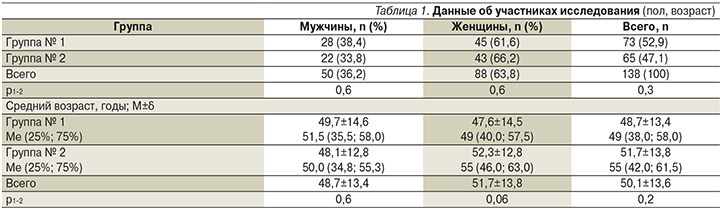
В исследование были включены 138 пациентов с подтвержденной инфекцией H. pylori. Группы больных, получавших классическую тройную схему и классическую тройную схему с добавлением ВТД, оказались сопоставимыми по возрасту и полу (табл. 1). Женщин было больше в обеих группах (61,6 и 66,2% соответственно). Средний возраст пациентов составил 50,1±13,6 года: 48,7±13,4 – в первой группе и 51,7±13,8 – во второй (р=0,2).
Большая часть (68,8%) пациентов страдали хроническим неатрофическим антральным гастритом, 27 (19,6%) больным был диагностирован хронический атрофический гастрит. У 14 пациентов была выявлена язвенная болезнь, в т.ч. у 11 (8%) с локализацией в 12-перстной кишке и у 3 (2,2%) – в желудке. Гастритом культи желудка (резекция по поводу осложненного течения язвенной болезни желудка) страдали 2 (1,4%) человек. У всех пациентов болезнь была ассоциирована с инфекцией H. pylori.
У 35 (25,4%) больных отягощенной оказалась наследственность: в 20,3% случаев по разным злокачественным новообразованиям, в 5,1% – по раку желудка.
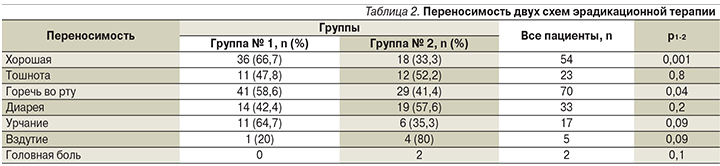
Для улучшения контроля за приверженностью лечению все пациенты вели дневник, где отмечали нежелательные явления, связанные с приемом препаратов. Три человека прекратили лечение (1 – в первой группе, 2 – во второй: у двух больных возникла аллергическая реакция, проявившаяся кожной сыпью, одна пациентка прекратила прием препаратов по личным мотивам). В целом отсутствие нежелательных явлений, связанных с приемом препаратов, наблюдалось у 54 (39%) па- циентов, чаще в первой группе (66,7 против 33,3%; р=0,001; табл. 2). Самым частым побочным симптомом при проведении эрадикации была горечь во рту (50,7%). Необходимо отметить, что этот симптом встречался значимо реже у пациентов, принимавших тройную схему с добавлением ВТД (Улькавис), по сравнению с классической тройной терапией (41,4 против 58,6%; р=0,04).
Диарея на фоне эрадикационной терапии, не потребовавшая отмены лечения, отмечена в 42,4% случаев в первой группе и в 57,6% случаев – во второй (р=0,2; табл. 2). При этом у пациентов, принимавших ВДТ (Улькавис), продолжительность диареи была на 2–3 дня короче, чем у больных, получавших классическую тройную терапию (р=0,04).
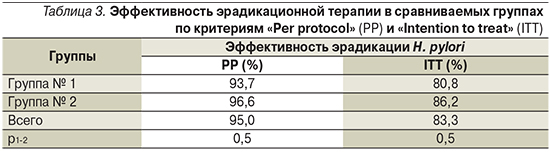
Контроль за эффективностью эрадикационной терапии был проведен через 8 недель после окончания лечения. К моменту завершения исследования результаты эрадикации были зарегистрированы у 121 пациента (87,8% от включенных в исследование и начавших лечение). Для контроля преимущественно (110 пациентов, 90,9%) использовали иммуноферментный анализ антигенов H. pylori в кале. Девяти (7,4%) проведено эндоскопическое исследование с быстрым уреазным тестом, С13-уреазный дыхательный тест выполнен 2 (1,7%) больным. Суммарно для 95% пациентов, выполнивших контрольное исследование, эрадикационная терапия оказалась успешной, в т.ч. в группе получавших классическую тройную терапию – в 93,7% случаев, для получавших схему с добавлением ВТД – в 96,6% (р=0,5) (табл. 3). Таким образом, показатель эффективности «Per protocol» (РР), т.е. рассчитанный на число получивших полный курс эрадикационной терапии и выполнивших контрольное исследование, оказался очень высоким в обеих группах. Показатель эффективности эрадикационной терапии «Intention to treat» (ITT) при расчете, т.е. на все 138 человек, включенных в исследование, оказался несколько ниже, но превышал отметку в 80% (83,3% – в общей группе, 80,8% – в группе классической тройной терапии и 86,2% – в группе с добавлением ВТД; р=0,5).
Обсуждение
Нами проведено исследование эффективности и переносимости сравнения двух схем эрадикационной терапии инфекции H. pylori с использованием мероприятий, повышающих эффективность лечения. Поскольку в европейской и российской европеоидной популяциях преобладают «быстрые» метаболизаторы ИПП (гомозиготы, нет мутаций CYP2C19) [3, 12], мы выбрали в качестве ИПП эзомепразол (Эманера), являющийся S-энантиомером (левый изомер) омепразола и обладающий большей, чем последний, биодоступностью [13]. Выбор дозы препарата был основан на многочисленных данных о повышении эффективности эрадикационной терапии при удвоении доз ИПП, а также о результатах наших предыдущих исследований, показавших убедительный антисекреторный эффект 40 мг Эманеры [14]. К популярным в последние годы мерам, повышающим эффективность эрадикационной терапии, относится и добавление к стандартной тройной терапии ВТД в дозе 240 мг 2 раза в сутки или 120 мг 4 раза в сутки [15–18]. Добавление препарата висмута к тройной терапии инфекции Н. pylori широко используется в Китае, а также в странах Европы и США [17, 18]. Терапия ВТД хорошо переносится больными, о чем свидетельствуют результаты мета-анализа с включением 26 рандомизированных исследований [19]. В настоящем исследовании обе схемы эрадикационной терапии (с ВДТ и без него) достаточно хорошо переносились пациентами. Ни один из побочных эффектов (кроме, аллергической реакции у двух пациентов) не потребовал отмены лечения. При этом такой частый симптом во время лечения, как горечь во рту, наблюдался значимо реже при добавлении в схему ВТД в виде Улькависа (р=0,005). Более того, у пациентов, принимавших Улькавис, продолжительность диареи на фоне лечения была на 2–3 дня короче, чем у больных группы, получавшей классическую тройную терапию (р=0,04).
Результаты ряда российских исследований свидетельствуют о повышении эффективности эрадикационной терапии инфекции Н. pylori при добавлении ВТД как к тройной, так и к последовательной схеме лечения [20, 22, 23]. Включение в схемы терапии ВТД, к которому не формируется резистентность H. pylori, оптимизирует действие антибиотиков, препятствуя развитию антибиотикорезистентности [17]. В настоящем исследовании эффективность лечения РР оказалась высокой в обеих группах (с ВТД и без него), составив 95% (93,7% для классической тройной и для 96,6% тройной терапии с добавлением Улькависа). Показатель эффективности эрадикации ITT был несколько ниже за счет не выполнивших контрольное исследование пациентов, однако даже при таком строгом подходе он оказался достаточно высоким (83,3% в общей группе, 80,8% в группе классической тройной терапии и 86,2% в группе с добавлением ВТД).
В международных и российских рекомендациях особое внимание уделяется приверженности пациентов лечению, в частности подробному инструктированию больных и контролю за соблюдением назначенного режима приема лекарственных средств [3, 12]. Отсутствие комплаенса может стать причиной неэффективности лечения даже при наличии чувствительных к назначенным антибиотикам штаммов Н. pylori [12]. Мы считаем, что высокий результат эрадикации в настоящем исследовании достигнут в т. ч. и за счет высокой приверженности пациентов лечению, обеспеченной их информированностью о целях и задачах лечения, возможных нежелательных явлениях, ведением дневника во время лечения и т. д.
Заключение
Классическая тройная схема с использованием удвоенной дозы генерического ИПП (Эманера) и усиленная генерическим препаратом ВТД (Улькавис) при условии высокой приверженности пациентов лечению демонстрирует высокую эффективность при меньшей частоте некоторых побочных эффектов и может быть рекомендована для широкого использования в лечебной практике.
Источник финансирования
Исследование выполнено в рамках государственного задания № 03242018-0001 «Эпидемиологический мониторинг состояния здоровья населения и изучение молекулярно-генетических и молекулярно-биологических механизмов развития распространенных терапевтических заболеваний в Сибири для совершенствования подходов к их диагностике, профилактике и лечению».



