Введение
На настоящий момент для лечения метастатического колоректального рака (мКРР) одобрена масса различных цитостатических и биологических препаратов, в т.ч. производные фторпиримидинов, оксалиплатин, иринотекан, антиангиогенные препараты, такие как бевацизумаб, аффлиберцепт, рамуцирумаб, регорафениб, а также анти-EGFR-моноклональные антитела цетуксимаб и панитумумаб [1, 2].
При этом стандарт терапии 1-й линии варьируется от комбинации цитостатического триплета с биологическим препаратом для пациентов с потенциально операбельным метастатическим заболеванием до монотерапии одним цитостатическим или анти-EGFR-препаратом для пациентов с диким типом генов KRAS, NRAS и несоответствующим состоянием по шкале ECOG – Eastern Cooperative Oncology Group [1, 2].
На настоящий момент принято считать, что максимальные показатели выживаемости больных мКРР до 18–20 месяцев достигаются при последовательном или комбинированном использовании всех активных цитостатических и биологических препаратов в зависимости от молекулярно-генетического статуса KRAS и NRAS [3, 3–6]. Как показано в крупных рандомизированных исследованиях 3-й фазы, проведение комбинированной терапии позволяет повышать частоту объективных ответов и время до прогрессирования, но не влияет на общую выживаемость (ОВ) относительно последовательной монотерапии [4, 6].
Стратегия лекарственной терапии мКРР описана в рекомендациях ESMO (EuropeanSociety for Medical Oncology), где в зависимости от сочетания клинических критериев все больные разделены на 4 принципиальные группы [2]. Так, пациенты с потенциально резектабельными метастатическими очагами должны получать режимы с максимально высокой частотой объективных ответов для достижения максимальной вероятности R0-резекции. Больным резектабельным заболеванием и с наличием симптомов, связанных с течением опухолевого процесса, также должны проводиться комбинированные режимы для максимального контроля размеров очагов и клинических симптомов. К последней категории относятся пациенты без выраженных клинических симптомов и потенциальной возможности для полной циторедукции. Таким пациентам следует оптимально проводить последовательную монотерапию с минимальной токсичностью.
Таким образом, когда целью лечения является контроль заболевания, абсолютно обоснованно проведение монотерапии с последующим рассмотрением полихимиотерапии (ПХТ) во 2-й линии [7].
Капецитабин служит предшественником 5-фторурацила для перорального применения. По сравнению с внутривенными режимами терапия капецитабином позволяет достигать более высоких показателей частоты объективных ответов и аналогичных с внутривенным лечением показателей времени до прогрессирования и общей продолжительности жизни на фоне относительно умеренной токсичности (Grade 3 и 4 в 38,1 и 3,0% соответственно) [8]. Наиболее частым видом побочных эффектов на фоне терапии капецитабином считаются ладонно-подошвенный синдром и диарея. Профиль токсичности позволяет закончить запланированное лечение без редукции дозы 34% пациентов. Кроме того, как было установлено в двух крупных рандомизированных исследованиях, редукция дозы не приводит к снижению эффективности терапии капецитабином [9].
К настоящему моменту получены результаты нескольких исследований по комбинированию капецитабина и биологических препаратов. Так, комбинация капецитабина и бевацизумаба приводит к более высокой частоте объективных ответов и большему времени до прогрессирования, но не увеличивает ОВ пациентов, при этом повышая риск сердечно-сосудистых побочных эффектов [10].
Применение анти-EGFR-терапии ограничено популяцией пациентов с диким типом генов KRAS и NRAS. Цетуксимаб в комбинации с капецитабином позволил пациентам с диким типом KRAS и противопоказаниями к терапии бевацизумабом достичь объективного ответа 48% пациентов. Время до прогрессирования на фоне данной комбинации составило 8,4 месяца. При этом высокая частота выраженных паранихиев потребовала редукции дозы капецитабина до 2000 мг/м2/сут [11].
На этом фоне монотерапия биологическими препаратами изучена существенно меньше. В литературе нам удалось найти данные только о двух исследованиях цетуксимаба в монорежиме. В исследовании A. Pessino et al. 39 пациентов получали терапию цетуксимабом в 1-й линии [12]. Несмотря на отсутствие селекции по мутационному статусу KRAS и NRAS, время до прогрессирования в данном исследовании составило 5 месяцев, частота объективных ответов – 11%, частота контроля заболевания – 44%. Длительность ответа на терапию в числовых значениях была существенно выше и составила 12,9 месяца. При этом профиль токсичности был несколько лучше, чем на фоне монотерапии фторпиримидинами. Во второе исследование включение пациентов производилось только на основании определения статуса KRAS [11]. Несмотря на относительно невысокую частоту мутаций NRAS, результаты могут быть скомпрометированы включением пациентов с мутацией этого гена.
Кроме селекции больных на основании молекулярных нарушений KRAS и NRAS относительно недавно были получены серьезные предпосылки к тому, чтобы предполагать неэффективность биологических анти-EGFR-препаратов для пациентов с опухолями правого фланга [13].
В связи со всем сказанным выше авторам представляется интересным исследовать вопрос монотерапии пациентов без мутаций KRAS, NRAS и BRAF анти-EGFR-препаратами в 1-й линии
Методы
Исследование было одобрено этическим комитетом ГБУЗ СПбКНпЦ СВМП(о).
Все процедуры исследования выполнялись после подписания информированного согласия каждым пациентом. В исследование включены больные гистологически подтвержденным метастатическим нерезектабельным раком толстой кишки, ранее не получавшие лекарственной терапии по поводу диссеминированного процесса, с общим соматическим статусом 0–1 балл по шкале ECOG (Восточная объединенная онкологическая группа) и без симптомов, обусловленных опухолевыми очагами.
В образцах опухолевой ткани, полученных при первичном хирургическом лечении или при биопсии, зафиксированных формалином и залитых парафином, в рамках лаборатории молекулярной онкологии НМИЦ онкологии им. Н.Н. Петрова МЗ РФ проведен молекулярный анализ наличия мутаций в генах KRas, NRas и BRAF с использованием методик, описанных ранее [14].
Пациентов, в опухолевой ткани которых не было выявлено мутаций в генах KRas, NRas и BRAF, включили в интервенционную фазу исследования, в которой проводилось лечение цетуксимабом внутривенно капельно в еженедельном режиме в стандартных дозировках (нагрузочная доза – 400 мг/м2, далее – 250 мг/м2 еженедельно). Перед каждым введением проводилась премедикация дифенгидрамином, согласно инструкции.
Перед первой инфузией препарата и далее каждые 2 недели оценивалась безопасность лечения, которая включала сбор анамнеза, физикальный осмотр, клинический и биохимический анализы крови. Токсичность терапии оценивалась согласно терминологии и критериям NCI CTC AE (National Cancer Institute Common Toxicity Criteria Adverse Events) v.4.03.
Оценки эффективности терапии проводили каждые 6 недель при помощи компьютерной томографии. Объективный ответ оценивался согласно критериям RECIST (Response evaluation criteria in solid tumours) v.1.1.
У всех пациентов оценивали показатели ОВ и выживаемости без прогрессирования (ВБП). ВБП оценивалась как период от дня первого введения препарата до прогрессирования по критериям RECIST v.1.1 или до смерти от любой причины. ОВ определялась как период от дня первого введения препарата до смерти от любой причины. Случаи, в которых связь с пациентом была утеряна и когда не удавалось получить информацию о статусе выживаемости, были цензурированы на дате последнего контакта с пациентом.
Статистические расчеты были основаны на двустадийном дизайне исследования Флеминга. На основании литературных данных ожидаемая 6-месячная ВБП на фоне терапии капецитабином была оценена как 30%. В качестве альтернативной гипотезы установлена 6-месячная ВБП для терапии цетуксимабом 50%. Для подтверждения альтернативной гипотезы с мощностью 80% и вероятностью ложно позитивного результата 5% было необходимо включение в исследование 36 пациентов.
В случае отсутствия прогрессирования в течение 6 месяцев у 11 из 19 пациентов, включенных в исследование на первом этапе, согласно статистическим расчетам, альтернативная гипотеза может считаться подтвержденной и исследование должно быть остановлено.
Так как в литературе отсутствуют данные об эффективности стандартной монотерапии фторпиримидинами больных мКРР, отобранных по молекулярным факторам (дикий тип KRas, NRas и BRAF), для независимой оценки эффективности проведен ретроспективный анализ молекулярных альтераций в опухолях, а также анализ клинических характеристик и результатов лечения пациентов, получавших монотерапию фторпиримидинами (5-фторурацилом, капецитабином, тегафуром) с 2013 по 2015 г. в ГБУЗ СПбКНпЦ СВМП(о).
Результаты исследования
Образцы опухолевой ткани 73 пациентов проспективно проанализированы на предмет наличия мутаций в генах KRas, NRas и BRAF. Средний возраст пациентов в выборке составил 68,9 года (54–85лет).
В 33 из 73 опухолей не обнаружено мутаций во всех 3 генах.
Клинические характеристики пациентов с диким типом генов KRas, NRas и BRAF не отличались от таковых в исследуемой группе в целом.
Эффективность и токсичность лечения в проспективном исследовании
Терапия цетуксимабом начата 21 из 33 пациентов. Медиана продолжительности терапии составила 3,9 месяца (95% доверительный интервал [ДИ] – 2,18–5,69 месяца). Два пациента выбыли из исследования до проведения оценок в связи с отзывом согласия (n=1) и потерей дальнейшего контакта (n=1). У 8 из 19 пациентов прогрессирование заболевания отмечено на фоне лечения, у 9 – после завершения периода лечения, у 1 пациента прогрессирования не зафиксировано до наступления смерти от причины, не связанной с опухолью.
У 11 (57,9%) из 19 пациентов через 6 месяцев после начала исследуемой терапии не отмечено признаков прогрессирования заболевания. В связи с тем, что таким образом альтернативная статистическая гипотеза оказалась подтвержденной на первом этапе исследования, набор пациентов был завершен.
Медиана ВБП составила 6,4 месяца (95% ДИ – 4,31–8,39 месяца).
На фоне лечения не наблюдалось ни одного полного регресса опухоли.
У 2 (10,5%) из 19 пациентов зарегистрирован частичный регресс. Для 11 из 19 больных максимальным эффектом лечения стала стабилизация заболевания. Таким образом, частота контроля заболевания составила 68%. Медиана продолжительности ответа пациентов, которыми был достигнут контроль заболевания, составила 7,3 месяца. Медиана ОВ пациентов оказалась равной 14,9 месяца (95% ДИ – 13,1–16,7 месяца).
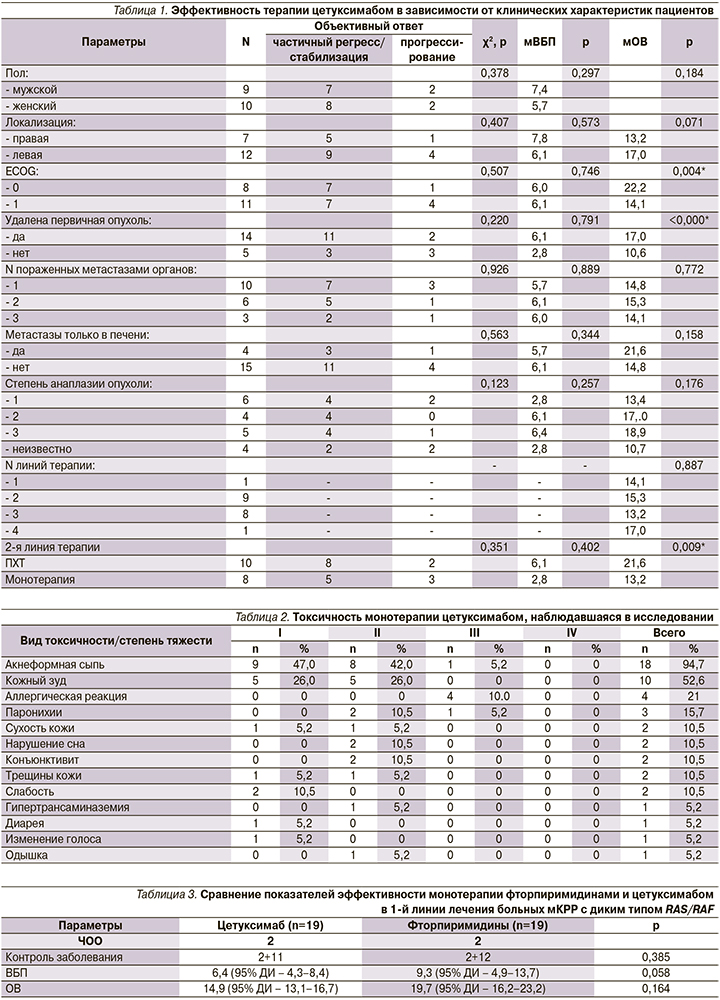
Подгрупповой анализ не показал статистически значимой зависимости ВБП или достижения контроля заболевания от каких-либо клинических характеристик (табл. 1).
ОВ оказалась достоверно выше у пациентов с общим состоянием по шкале ECOG 0 баллов на момент начала лечения по сравнению с 1–2 баллами (22,2 против 14,1 месяца; p=0,004), у пациентов с удаленной ранее первичной опухолью (17,0 против 10,7 месяца; p=0,015) и у пациентов, получавших в качестве последующих линий лечения ПХТ, по сравнению с получавшими монотерапию фторпиримидинами (21,6 против 13,2 месяца; p=0,009).
У всех пациентов в процессе терапии наблюдались нежелательные явления (НЯ). Ни одно из НЯ не привело к смерти. Все наблюдавшиеся токсические реакции были обратимыми, и их тяжесть снижалась до 1-й степени не более чем за 3 недели.
НЯ 3-й степени наблюдались у 6 (31,5%) из 19 пациентов: 4 аллергические реакции, 1 акнеформная кожная сыпь, 1 паронихия. У 2 пациентов доза цетуксимаба была снижена на 25% по причине токсичности на 25%. Наиболее часто наблюдавшимися видами токсичности любой степени были акнеформная сыпь, кожный зуд, паронихии. Другие виды НЯ, не относящиеся к кожной токсичности, были легкими и наблюдались существенно реже. Среди них: нарушение сна, слабость, гипертрансаминаземия, диарея, изменение голоса и одышка (табл. 2).
Ретроспективный анализ эффективности монотерапии фторпиримидинами в 1-й линии лечения при мКРР с диким типом RAS/RA, сравнение с монотерапией цетуксимабом
Проведен ретроспективный анализ медицинских карт 3200 пациентов, получавших лечение по поводу колоректального рака в ГБУЗ СПбКНпЦ СВМП(о) с 2013 по 2015 г. Из пациентов, получавших 1-ю линию химиотерапии по поводу мКРР с адекватной оценкой эффективности, гистологические препараты были доступными для 241 (из них 95 получали различные режимы монотерапии). Проведен молекулярный анализ этих образцов. У 57 пациентов в опухоли не обнаружено мутаций KRas, NRas и BRAF. Для анализа отобраны 19 из этих случаев с нерезектабельным мКРР, в которых в качестве 1-й линии терапии применялись фторпиримидины в монорежиме. Основные клинические характеристики этой выборки пациентов не имели статистически значимых отличий от таковых больных, включенных в проспективное исследование.
При сравнении ВБП и ОВ на фоне монотерапии фторпиримидинами и цетуксимабом статистически значимых различий не выявлено (табл. 3).
Медиана продолжительности ответа также не различалась между группами и составила 9,7 (95% ДИ – 5,0–14,3 месяца) и 7,3 месяца (95% ДИ – 4,9–9,9 месяца) для терапии фторпиримидинами и цетуксимабом соответственно.
Обсуждение
Проведено проспективное исследование 2-й фазы терапии цетуксимабом в монорежиме пациентов с нерезектабельным мКРР без мутаций в генах KRas, NRas и BRAF, у которых не наблюдалось симптоматических проявлений опухоли. Согласно спланированному дизайну, исследование преждевременно остановлено, т.к. у 11 (57,9%) из 19 пациентов, включенных на первом этапе, не было зафиксировано прогрессирования заболевания через 6 месяцев от начала терапии, что было установлено как критерий подтверждения гипотезы об эффективности лечения.
Медиана ВБП в обогащенной на основании молекулярных факторов группе составила 6,4 месяца, что превышало показатели, ранее опубликованные в международных источниках для монотерапии цетуксимабом неселектированных пациентов (5 месяцев) или пациентов, отобранных на основании наличия экспрессии рецептора эпидермального фактора роста в опухоли (2,9 месяца), а также в исследовании ASPECT ранее получавших лечение пациентов, отобранных на основании статуса RAS [11, 12, 15].
ОВ в исследуемой группе пациентов существенно зависела от последующих линий химиотерапии. Пациенты, в дальнейшем получавшие в качестве 2-й линии ПХТ (например, по схемам FOLFOX или FOLFIRI), имели преимущество в выживаемости по сравнению с больными, получавшими монотерапию фторпиримидинами (капецитабин, тегафур), что идет в разрез с данными других исследований, показавших, что последовательное применение цитостатиков не уступает применению комбинированной терапии в ОВ. Эти результаты могут быть скомпрометированы малым числом пациентов в нашем исследовании, а также неслучайным подходом к выбору терапии 2-й линии (пациенты с лучшим объективным статусом и как следствие – с лучшим прогнозом получали ПХТ).
Частота токсичности 3-й степени тяжести, наблюдавшаяся в нашем исследовании, была сопоставимой с монотерапией фторпиримидинами, но превышала показатели, описанные в ранее опубликованной литературе для монотерапии цетуксимабом.
На основании полученных результатов можно предположить, что селекция на основании дикого типа 3 генов (KRas, NRas и BRAF) недостаточна для отбора наиболее чувствительных к терапии цетуксимабом опухолей. Тогда как ВБП у 4 пациентов в нашем исследовании превысила 9 месяцев, в то же время у 6 больных наблюдалось прогрессирование заболевания при первой же оценке эффекта.
Заключение
Таким образом, даже среди селектированной на основании современных молекулярных маркеров группы пациентов существенная часть не отвечает на терапию цетуксимабом. Более глубокий и сложный молекулярный анализ биологии опухоли необходим для прецизионного отбора пациентов, у которых высока эффективность лечения цетуксимабом.



