Введение
Мигрень – распространенная форма первичной головной боли (ГБ), значимо влияющей на жизнь людей, ею страдающих. Данные эпидемиологических исследований во всем мире подтвердили высокую распространенность мигрени, социально-экономические последствия и индивидуальное бремя для пациентов и их семей. В 2015 г. мигрень заняла третье место среди причин нетрудоспособности в мире у мужчин и женщин в возрасте до 50 лет [10]. Столь значимое влияние мигрени на качество жизни продиктовало необходимость поиска новых эффективных средств для ее лечения.
Первым доступным для широкого применения классом препаратов для специфической профилактики мигрени, разработанных с учетом новых открытий в патогенезе этого заболевания, стали анти-CGRP-моноклональные антитела.
Эренумаб – это человеческое моноклональное антитело (мАТ), являющееся антагонистом рецептора кальцитонин ген родственного пептида CGRP (Calcitonin gene-related peptide) [8]. Значимые различия с плацебо, стойкий клинический эффект и высокий уровень безопасности эренумаба убедительно показаны в нескольких крупных плацебо-контролируемых исследованиях [6, 7, 11]. Не менее важную информацию несут и исследования эффективности и безопасности препарата в условиях обычной клинической практики RWS (Real World Studies), где встречаются пациенты с различными формами мигрени, различной чувствительностью к антимигренозной терапии, а также с сопутствующими и коморбидными заболеваниями.
В канадское исследование [4] вошли 95 пациентов с эпизодической (ЭМ) и хронической (ХМ) мигренью, ранее не отвечавших на лечение профилактическими средствами 2–3-го классов. Доза эренумаба выбиралась на усмотрение врача; большинство (93,7%) пациентов начали с дозы 140 мг ежемесячно. На 12-й неделе 33,7% пациентов (из них 26,6% с ХМ и 48,4 с ЭМ) достигли ≥50%-ного снижения дней с мигренью в месяц, а на 24-й неделе этот показатель составил 34,9% (34,5% с ХМ и 35,5% с ЭМ). Пациенты отметили более мягкое течение приступов и улучшение функциональной активности.
Во французское исследование вошли 144 пациента, включенных в Федеративную госпитально-университетскую программу FHU (Federation Hospitalo-Universitaire InovPain) [12]. Стартовая доза эренумаба составляла 70 или 140 мг в месяц на усмотрение лечащего врача, была возможность увеличения дозировки при хорошей переносимости. Число дней с мигренью в месяц опрашивал лечащий врач на каждом из ежемесячных визитов и вносил эти данные в регистр. Ценность исследования заключалась в том, что пациенты на каждом из визитов заполняли шкалы, оценивавшие влияние мигрени на их состояние. Курс лечения составил 12 месяцев. Доли респондеров с 50%-ным ответом на лечение составили 52,9% через 3 месяца, 58,5% через 6, 57,0% через 9 и 58,8% через 12 месяцев. Согласно опроснику общего впечатления пациента PGIC (Patient Global Rating of Change in Migraine), к 12-му месяцу лечение сочли эффективным 65,7% участников исследования. На протяжении курса терапии достоверно уменьшалась тяжесть мигрени по шкале HIT-6. Таким образом, исследование подтвердило устойчивый эффект эренумаба. Среди побочных эффектов отмечены покраснение/боль в месте инъекции (30% пациентов), констипация (15,7%), мышечный спазм (1,4%), аллопеция (0,7%).
Значимый опыт использования эренумаба показан и нашими коллегами. Была проведена апробация препарата в Научном центре неврологии [2]. Лечение эренумабом в ежемесячной дозе 70 мг получали 35 пациентов с частой ЭМ. Через 12 недель в исследуемой группе отмечено уменьшение числа дней с ГБ с 10,3±2,3 до 5,0±3,6 (p<0,000001). Частота приступов мигрени уменьшилась на 50% у 60% пациентов. Отмечена хорошая переносимость препарата; склонность к запорам была у 25,7% пациентов. В другое открытое исследование, проведенное в РФ [1], вошли 33 пациента с ЭМ и ХМ. За 1-й месяц терапии 50%-ное уменьшение дней с ГБ отмечено у 48% пациентов, за 2-й – у 53%, за 3-й – у 51,5%. Клиническое улучшение также заключалось в снижении ситуативной тревожности, уменьшении числа дней с использованием триптанов и анальгетиков. Лечение переносилось хорошо: из 33 пациентов у одного отмечалась преходящая констипация.
В исследовании Е.В. Екушевой и соавт. [3] вошли 48 пациентов с ХМ, получавших 70 мг эренумаба ежемесячно. Среднее число дней с ГБ к 12-й неделе снизилось от 24 до 10, число дней с мигренью – от 14 до 5 в месяц.
Немаловажным аспектом является эффективность и безопасность длительного использования эренумаба. Так, в открытое 52-недельное исследование, проведенное в Датском центре ГБ [5], были включены 300 пациентов с ХМ, не ответивших по крайней мере на один препарат из группы антиконвульсантов и один препарат из группы антигипертензивных средств, получивших хотя бы одну дозу эренумаба. Начальная доза эренумаба была 70 мг ежемесячно. Доля пациентов, достигших снижения среднего числа дней с мигренью в месяц на 30% и более через 12 недель лечения, составила 71,4%. Снижение числа дней с мигренью более чем наполовину отмечалось у 56,3% пациентов. В течение курса лечения у части пациентов доза эренумаба была повышена до 140 мг ежемесячно. К 52-й неделе терапии у 34% всех пациентов, получавших дозу 75 мг, сохранялось ≥30%-ное снижение числа дней с мигренью в месяц. При повышении дозы эренумаба до 140 мг ежемесячно к 52-й неделе доля пациентов с ≥30%-ным снижением дней с мигренью в месяц составила 82,1%. Побочные эффекты отмечены у 73,3% пациентов, наиболее частыми из которых были констипация, тошнота и общая слабость.
Похожее исследование было проведено в США [14], в котором 177 пациентов с рефрактерной ХМ получали эренумаб в дозе 70 или 140 мг в месяц. Клинически значимое улучшение ≥30% отмечалось у 61,6% участников. На длительной терапии (17–30 месяцев) оставались 54,8% пациентов исследуемой группы, отмечавшие отчетливое клиническое улучшение.
В Канаде был проанализирован регистр пациентов с ХМ (13 352 человека), получавших эренумаб в начальной дозе 70 (47,7%) или 140 (52,3%) мг [10]. В течение года и более оставались на терапии 71% пациентов. Среди пациентов, получавших начальную дозу 70 мг, 59,3% были переведены на дозу 140 мг. Лица (n=67) из регистра пациентов с мигренью в США согласились принять участие в опросе об удовлетворенности лечением эренумабом, 71,6% оценили лечение как эффективное [13].
Методы
Целью нашего исследования было изучить эффективность, безопасность, а также паттерны применения эренумаба в условиях рутинной практики специализированного центра ГБ в Российской Федерации.
В открытое ретроспективное несравнительное исследование вошли 126 пациентов старше 18 лет, наблюдавшихся в Университетской клинике головной боли (Москва), получивших по меньшей мере 3 инъекции эренумаба и регулярно заполнявших дневник ГБ Мигребот за 1 месяц до начала терапии и в течение всего курса лечения. Диагноз мигрени ставился на основании критериев Международной классификации ГБ III (МКГБ-III, 2018). Пациенты сообщали лечащему врачу о всех побочных эффектах.
Первичным показателем эффективности была динамика уменьшения числа дней с мигренью на 12-й неделе терапии по сравнению с числом дней с мигренью до начала терапии. Другими показателями эффективности была доля пациентов с ≥50%-ной редукцией дней с мигренью через 12 недель терапии.
Для статистического анализа испо-льзовался программный пакет SPSS 10.0. Для числовых характеристик симптомов и признаков использовались описательные статистики – анализ частоты и средние значения. Численные показатели приведены в формате «среднее±среднеквадратичное отклонение». Все показатели проверялись на нормальное распределение по критерию Колмагорова–Смирнова. При условии нормального распределения использовались параметрические методы. Если нормальное распределение отсутствовало, то использовались непараметрические методы. Для оценки динамики показателя использовался Т-критерий для парных выборок. Статистически значимыми считались различия при p<0,05. Для корреляционного анализа (взаимосвязь ответа на терапию с различными факторами) использовался коэффициент Спирмана.
Результаты
Средний возраст пациентов составил 40,3±9–10 лет (19–67), преобладали женщины (Ж – 111, М – 15). Средняя продолжительность анамнеза мигрени составила 24,8±12,4 года (разброс от 7 до 52 лет). Были включены пациенты с различными типами мигрени: ЭМ отмечалась у 29 (23%), ХМ – у 97 (77%) пациентов. Мигрень с аурой отмечена у 17 (13,5%), лекарственно-индуцированная ГБ (ЛИГБ) – у 58 (46%). Среди женщин у 58 (52,3%) мигрень была менструально-ассоциированной. Среди коморбидных заболеваний у 67 (53,2%) отмечалось депрессивное расстройство, установленное психиатром.
Перед началом терапии эренумабом у 50 (39,7%) был неуспех приема двух и более классов пероральных средств для профилактики мигрени и/или ботулинотерапии и/или использования другого анти-CGRP-моноклонального антитела. Для большинства пациентов исследуемой группы (74,6% [n=94]) назначение эренумаба было второй или третьей линией терапии мигрени, однако почти четверть пациентов начали лечение мигрени с эренумаба.
Начальная дозировка эренумаба составила 70 мг в месяц. На усмотрение врача доза могла повышаться до 140 мг в месяц. Доза 70 мг использовалась у 116 (92,1%) пациентов, у 10 (7,9%) в процессе лечения доза повышалась до 140 мг в месяц.
Динамика числа дней с мигренью до начала терапии и через 12 недель терапии представлена на рисунке.
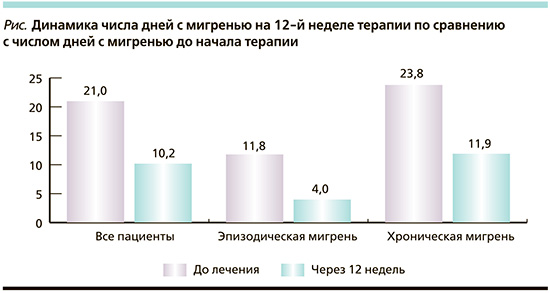
До лечения число дней с мигренью в исследуемой группе составляло 21,1±7,6, через 12 недель этот показатель составил 10,2±8,3 дня (р<0,0001). Среди пациентов с ЭМ число дней с мигренью до терапии было 11,8±3,7, а через 12 недель – 4,0±3,3 (р<0,0001).
В подгруппе ХМ перед лечением число дней с мигренью до терапии составило 23,8±6,2, а через 12 недель – 11,9±8,5 (р<0,0001).
Значимая динамика наблюдалась уже в течение первого месяца. Число дней с мигренью во всей группе уменьшилось от 21,1±7,6 до 11,0±8,3 через 4 недели (p<0,0001) и до 10,4±8,5 через 8 недель терапии (р=0,003). В подгруппе ЭМ число дней с мигренью уменьшилось от 11,8±3,7 до 4,5±3,8 через 4 недели (p<0,0001) и до 4,2±3,1 через 8 недель (р=0,2). В подгруппе ХМ число дней с мигренью уменьшилось от 23,8±6,2 до 12,9±8,3 через 4 недели (p<0,0001) и до 12,2±8,7 через 8 недель (р=0,006). Таким образом, можно говорить о быстром наступлении эффекта эренумаба и накоплении его в период курса лечения, в особенности в группе ХМ.
Доля пациентов с ≥50%-ной редукцией дней с мигренью через 12 недель терапии составила 68,3% (n=86), с ≥75%-ной редукцией – 32,5% (n=41). Доля пациентов с полной (100%) редукцией дней с мигренью была 5,6% (n=7). В группе ЭМ доля пациентов с ≥50%-ной редукцией составила 86,2% (n=25), с ≥75%-ной – 41,4% (n=12).
В группе ХМ доля пациентов с ≥50%-ной редукцией составила 62,9% (n=61), с ≥75%-ной – 29,9% (n=41).
Ответ на терапию (≥50%-ная редукция дней с мигренью) имел обратную корреляцию с наличием депрессии (р=0,003) и чувствительностью к триптанам (р<0,0001).
При недостаточном эффекте 35 (87,5%) пациентов-нереспондеров перевели на терапию другими препаратами. Остальным пациентам либо проводили эскалацию дозы, либо комбинировали с ботулинотерапией на усмотрение лечащего врача.
Необходимо отметить хорошую переносимость эренумаба в дозе 70 мг 1 раз в месяц. Лишь у одного пациента отмечалась склонность к констипации, что заставило отказаться от терапии. Другие пациенты активных жалоб на проблемы, связанные с функцией желудочно-кишечного тракта, не предъявляли. Еще у одного пациента отмечалось повышение артериального давления до 140–150/90 мм рт.ст., ранее не наблюдаемое.
Обсуждение
Наше исследование дополняет российский опыт использования препарата эренумаб и демонстрирует его высокую эффективность в лечении различных форм мигрени. Важно отметить, что полученные нами данные корреспондируют с результатами крупных открытых исследований в условиях рутинной клинической практики [5, 14]. Так, 53–79% различных популяций положительно ответили на терапию эренумабом в течение 3 месяцев. В нашем исследовании в условиях Специализированного центра лечения ГБ доля респондеров составила 68%. Показана высокая эффективность пациентов с эпизодической и хронической формами мигрени. Следует подчеркнуть, что у большинства из этих пациентов ранее были неудачные попытки лечения мигрени пероральными препаратами, ботулиническим токсином или другими анти-CGRP мАТ.
Ценным клиническим эффектом можно считать значимое действие эренумаба начиная с первого месяца терапии. Отсутствие необходимости титрования и длительного ожидания накопления эффекта может быть ценным для пациентов в состоянии прехронической мигрени или с частыми мигренозными статусами. Приступы менструально-ассоциированной мигрени известны своей тяжестью и сложностью купирования. Около половины женщин исследуемой группы отмечали менструально-ассоциированные приступы и испытывали значимое облегчение при использовании эренумаба.
В отличие от других исследователей в нашей работе представлена низкая встречаемость побочных эффектов. Данная особенность может быть связана как с использованием низкой дозы эренумаба (70 мг в месяц), так и с особенностями популяции пациентов, не связывающих незначительные для них симптомы с инъекциями препарата.
Депрессия, а также нечувствительность к триптанам были предиктором худшего ответа на анти-CGRP-тера-пию и в ранее проведенных исследованиях. Коррекция депрессивных расстройств – один из путей повышения эффективности анти-CGRP-терапии. Пациенты с отсутствием ответа на эренумаб должны быть скринированы на наличие депрессии и при положительном результате консультированы у психиатра или психотерапевта.
Заключение
Таким образом, эренумаб является эффективным и безопасным средством для профилактики мигрени в условиях повседневной практики и может быть рекомендован пациентам с различными формами мигрени.



