Введение
Резистентную артериальную гипертензию (АГ) традиционно определяют по клиническому (или «офисному») артериальному давлению (АД)≥140/90 мм рт.ст. у пациентов, получавших не менее трех антигипертензивных препаратов, включая ингибитор ренин-ангиотензин-альдостероновой системы (РААС), блокатор кальциевых каналов и диуретик в максимально переносимых дозах [1]. В руководстве по АГ Ассоциации кардиологов США к резистентной форме АГ относят АГ у неконтролируемых пациентов, принимающих не менее четырех антигипертензивных препаратов [2]. Спиронолактон является четвертым препаратом, добавляемым к фармакотерапии, в которой уже используется диуретик. Эксперты определяют механизмы, лежащие в основе резистентной АГ, как персистирующая избыточная внутрисосудистая задержка жидкости, являющаяся основным триггером (рис. 1) [2].
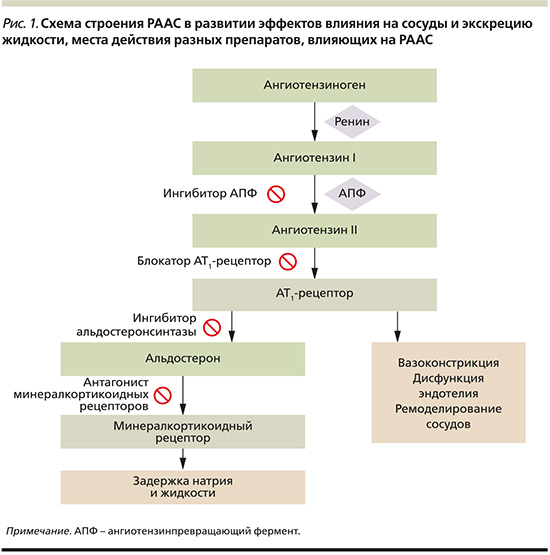
Проблема резистентной АГ сохраняет актуальность в современных условиях ввиду устойчивой распространенности в популяции пациентов с АГ и существующих дискуссий по тактике лечения, в т.ч. медикаментозных и немедикаментозных подходов (например, почечная денервация). По данным последнего мета-анализа 2019 г., объединившего более 90 популяционных исследований и когорту в 3,2 млн пациентов, распространенность резистентной АГ составляет 10,3% [3]. Пациенты с резистентной АГ чаще имеют факторы риска сердечно-сосудистых заболеваний, включая сахарный диабет, ХБП и высокий прогнозируемый 10-летний риск, что повышает риск сердечно-сосудистых осложнений и смертности. Так, по данным исследования в США, в когорте пациентов с резистентной АГ выявлен более высокий риск сердечно-сосудистой смертности (относительный риск [ОР]=1,47), в т.ч. при контролируемой (ОР=1,66) и неконтролируемой (ОР=1,46) по уровню АД [4]. Данные о более высокой сердечно-сосудистой смертности согласуются с результатами исследования в Корее пациентов с резистентной АГ (ОР=1,62) [5].
Роль альдостерона в развитии резистентной АГ
Альдостерон играет важную роль при АГ, и около 15% пациентов имеют аномально высокое соотношение альдостерон/ренин, а у пациентов с резистентной АГ эта доля возрастает примерно до 25%. Ранее было установлено, что альдостерон, минералокортикоид, является важным фактором в развитии резистентной АГ, при этом первичный альдостеронизм присутствует примерно у 20% пациентов данной категории [6]. Таким образом, у пациентов с резистентной АГ обнаружены более высокие суточные уровни альдостерона в моче по сравнению с пациентами с контролируемой АГ.
Антагонисты минералокортикоидных рецепторов
Спиронолактон – антагонист минералокортикоидных рецепторов рекомендован в качестве препарата 4-й линии препаратов и способен снижать САД/ДАД (диастолическое АД) на 20–25/10–12 мм рт.ст. у пациентов с резистентной АГ с первичным альдостеронизмом и без него. Реакция АД на спиронолактон примерно в 2 раза выше, чем на другие классы антигипертензивных препаратов в случаях резистентной АГ, но он может вызывать реактивное повышение уровня циркулирующего ренина и альдостерона вследствие механизма «обратной связи», снижая эффективность лечения. Частота применения антагонистов минералокортикоидных рецепторов (спиронолактон, эплеренон) при резистентной АГ мала и не превышает 9% [7].
Побочные эффекты спиронолактона включают гиперкалиемию, снижение синтеза тестостерона, гинекомастию, болезненность молочных желез, нарушения менструального цикла и кровотечения в постменопаузе, что связано с нецелевой блокадой нескольких рецепторов стероидных гормонов [8]. Ввиду большей селективности действия на минералокортикоидные рецепторы и минимального связывания с рецепторами прогестерона и андрогенов для эплеренона побочные эффекты, такие как гинекомастия и вагинальные кровотечения, менее выражены [9].
Ингибиторы альдостеронсинтазы
Чтобы противодействовать развитию эффектов на половые гормоны, был применен другой подход – прямое воздействие на синтез альдостерона вместо блокирования его рецептора.
Осилодростат – первый пероральный биодоступный ингибитор стероидогенеза надпочечников, был разработан для снижения уровня альдостерона в сыворотке крови и контроля АГ [10]. Он блокирует фермент альдостеронсинтазу, который контролируется регуляцией транскрипции гена CYP11B2 (рис. 2). Однако вскоре выяснилось, что осилодростат также ингибирует 11β-гидроксилазу – другой фермент, кодируемый геном CYP11B1 и участвующий в синтезе кортизола (рис. 2), что приводит к снижению уровня кортизола в сыворотке. Таким образом, фокус применения осилодростата сместился на лечение гиперкортицизма при Болезни Кушинга [11].

Таким образом, сходство альдостеронсинтазы и 11-β-гидроксилазы затрудняет поиск специфического ингибитора, который избирательно воздействует на альдостеронсинтазу.
Бакдростат (Baxdrostat) – новый селективный ингибитор альдостеронсинтазы без воздействия на 11β-гидроксилазу, предназначенный для лечения заболеваний, связанных с повышенным уровнем альдостерона (рис. 3) [12].
В доклинических исследованиях бакдростат показал высокую селективность in vitro в отношении альдостеронсинтазы по сравнению с 11β-гидроксилазой (индекс селективности – 100:1) [12]. Однократные дозы бакдростата на клеточных линиях и экспериментальным животным в 360-кратном диапазоне доз снижали уровень альдостерона в плазме и моче, демонстрируя возможность полностью подавлять выработку альдостерона, не влияя на выработку кортизола.
У здоровых добровольцев в клиническом исследовании 1-й фазы (n=54) бакдростат проявил свойства сильнодействующего селективного конкурентного ингибитора альдостеронсинтазы с дозозависимым снижением уровня альдостерона в плазме крови в диапазоне доз 1,5–10,0 мг. По данным исследований дозозависимости, высокое подавление синтеза альдостерона достигается при однократном приеме 3 мг бакдростата и практически полное подавление синтеза альдостерона при приеме 10 мг [13].
Бакдростат быстро всасывается, достигая максимальной концентрации через 4 часа после приема. Средний период полувыведения составил 26–31 час и ≈7% дозы выводится в неизменном виде с мочой. В равновесном состоянии экспозиция бакдростата была примерно в 2–2,5 раза выше, чем после однократного приема [13].
Недавно завершилось клиническое исследование 2-й фазы BrigHTN по изучению эффективности и безопасности применения бакдростата пациентами с резистентной АГ, имеющих уровень АД выше 130/90 мм рт.ст. [14, 15]. В исследовании участвовали 274 пациента, которые случайным образом были распределены в группу плацебо, бакдростата 0,5 мг, 1,0 и 2,0 мг 1 раз в сутки в течение 12 недель. Первичной конечной точкой было изменение САД к 12-й неделе по сравнению с группой плацебо. Дозозависимые изменения САД составили на -20,3 мм рт.ст., -17,5, -12,1 и -9,4 мм рт.ст. в группах 2,0 мг, 1,0, 0,5 мг и плацебо соответственно. Разница в изменении САД между группой, принимавшей 2 мг, и плацебо составила -11,0 мм рт.ст. (р=0,003) (рис. 4). Исследование было остановлено досрочно из-за подавляющей эффективности препарата: через 12 недель после рандомизации бакдростат в дозах 1 и 2 мг значительно снижал САД по сравнению с плацебо (что соответствовало основному результату исследования). Вторичный исход (различия в диастолическом АД) наблюдался при дозе 2 мг.
Анализ побочных эффектов, по мнению исследователей, не выявил ни одного серьезного побочного эффекта, связанного с приемом бакдростата, и не было случаев недостаточности коры надпочечников. Связанное с бакдростатом повышение уровня калия до 6,0 ммоль/л и выше произошло у двух пациентов, но это повышение не повторялось после отмены и возобновления приема препарата.
Заключение
Таким образом, поиск новых эффективных и безопасных блокаторов альдостерона продолжается. Применение блокаторов альдостерона при лечении АГ невелико и в большинстве руководств по лечению АГ им отводится место в качестве терапии четвертой или пятой линии при резистентной форме. Дополнительные исследования, демонстрирующие защиту органов-мишеней у пациентов с АГ, могут проложить путь к более широкому использованию ингибиторов альдостеронсинтазы.



