Введение
Прогрессирующее течение сахарного диабета 2 типа (СД2) часто приводит к необходимости назначения комбинированной терапии пероральными сахароснижающими препаратами [2], и в конечном итоге для поддержания уровня глюкозы может потребоваться инсулин в качестве монотерапии или в сочетании с другими сахароснижающими средствами [3].
Ингибиторы натрий-глюкозного котранспортера-2 (SGLT2) служат хорошим дополнением к монотерапии метформином или комбинации инсулина с метформином для лечения пациентов с СД2. В отличие от инсулина ингибиторы SGLT2 не вызывают гипогликемии при применении в качестве монотерапии или при совместном применении с другими сахароснижающими препаратами [4]. Кроме этого лечение ингибиторами SGLT2 также связано с умеренным снижением массы тела (МТ) и артериального давления (АД) [5].
Для пациентов с продолжительным течением диабета и высоким риском повреждения органов-мишеней при сердечно-сосудистых заболеваниях и хронической болезни почек (ХБП) большое значение ингибиторов SGLT2 [6, 7] связано со снижением риска сердечно-сосудистых событий, включая госпитализацию по поводу сердечной недостаточности и сохранения функции почек [8—14].
Эффекты ингибитора SGLT2 эртуглифлозина на кардиоренальные исходы у пациентов с СД2 и атеросклеротическими сердечно-сосудистыми заболеваниями (АСССЗ) были оценены в исследовании VERTIS CV [14-16]. В VERTIS CV около 50% пациентов принимали инсулин на исходном уровне. В других исследованиях с ингибиторами SGLT2 и оценкой сердечно-сосудистых исходов от 40 до 50% пациентов исходно получали инсулин [8-10, 14]. Эти проценты предполагают, что значительному числу пациентов с СД2 с высоким риском развития АСССЗ и/или ХБП и тем, кто использует инсулин в реальных клинических условиях, может потребоваться дополнительный гликемический контроль.
Исследования показали, что около двух третей пациентов с СД2, получавших базальный инсулин, терпели неудачу в достижении оптимального гликемического контроля через 12 месяцев лечения [17-19], что предполагает потребность в дополнительных терапевтических стратегиях.
Основной целью исследования было определить влияние эртуглифлозина по сравнению с плацебо на уровень гликозилированного гемоглобина (HbA1c) и оценить безопасность и переносимость эртуглифлозина. Вторичные цели заключались в оценке влияния эртуглифлозина на гликемию плазмы натощак (ГПН), массу тела, долю пациентов с HbA1c<7% (53 ммоль/ моль), систолическое АД (САД), диастолическое АД (ДАД) и дозу инсулина. Оценки кардиоренальных конечных точек, требующие большей продолжительности наблюдения, не служили целью дополнительного исследования и о них сообщалось ранее [14].
Методы
Исследование VERTIS CV было многоцентровым рандомизированным двойным слепым плацебо-контролируемым с параллельными группами, ориентированным на основные сердечно-сосудистые исходы [14, 20]. В исследование VERTIS CV были включены пациенты в возрасте >40 лет с СД2, уровнем HbA1c 7,0-10,5% (53-91 ммоль/моль), которые имели установленный диагноз АСССЗ с вовлечением коронарной, цереброваскулярной и/или периферической артериальной системы. Конкретные критерии включения и исключения, а также общий дизайн исследования VERTIS CV были опубликованы ранее [20].
В 18-недельном дополнительном исследовании ВЕРТИС CV оценивали гликемический контроль, кардиометаболическую эффективность и безопасность эртуглифлозина, добавленного к терапии инсулином, у пациентов с СД2 и АСССЗ, получавших инсулин в дозе >20 ЕД/сут в комбинации с или без метформина в дозе >1500 мг/ сут. Никакие другие сахароснижающие препараты не были включены. Протокол требовал, чтобы пациенты получали стабильную дозу инсулина в течение >8 недель до скрининга и поддержание той же дозы в течение 18-недельного периода дополнительного исследования, что позволило бы оценить гликемические эффекты эртуглифлозина. Общая суточная доза вариации инсулина ±10% в течение 8 недель до скринингового визита или в течение периода между скрининговым визитом и рандомизацией была разрешена и соответствовала критерию стабильной инсулинотерапии. Пациенты, использовавшие только прандиальный инсулин, были исключены. В течение 18 недель субисследования изменения фоновой сахароснижающей терапии не допускались, за исключением случаев, когда пациенты достигали предварительно определенных гликемических порогов лечения или испытывали клинически значимую гипогликемию.
Оценка эффективности проводилась на 0-й (исходный уровень), 6-й, 12-й и 18-й неделях. При каждом последующем посещении осуществлялся анализ лабораторных и антропометрических данных, собирались данные о достижении конечных точек и возникновении нежелательных явлений (НЯ) [1]. Заранее определенными НЯ, представляющими особый интерес (НЯ уровня 1), были генитальная микотическая инфекция (ГМИ) с гендерными различиями, инфекция мочевыводящих путей (ИМП), симптоматическая гипогликемия (событие с клиническими признаками, определенное исследователем как гипогликемия) и гиповолемия, НЯ уровня 2, т.е. которые не относились к уровню 1, но возникали у >4 пациентов в любой группе лечения. Другие интересующие НЯ включали документированную гипогликемию (эпизоды с уровнем глюкозы <70 мг/ дл/3,9 ммоль/л) и тяжелую гипогликемию (эпизоды, требующие медицинской или немедицинской помощи, независимо от уровня гликемии).
Размер выборки оценивали по снижению уровня HbA1c на 18-й неделе по сравнению с исходным уровнем с помощью продольного анализа данных (LDA - Linear Discriminant Analysis). Модель, включившая следующие переменные: визит (категория), лечение при посещении, исходную расчетную скорость клубочковой фильтрации (рСКФ) и использование метформина (да/нет). Для оценки доли пациентов с HbA1c<7,0% (53 ммоль/моль) использовали логистический регрессионный анализ на 18-й неделе. Статистическая модель включала длительность лечения, исходный уровень НЬА1с, использование метформина (да/нет) и исходную рСКФ.
Сравнительный анализ распределения события во времени для каждой дозы эртуглифлозина по сравнению с плацебо проанализирован с использованием логарифмического ранга. Снижение HbA1c по сравнению с исходным уровнем на 18-й неделе оценивали в ковариационной модели анализа повторных измерений в подгруппах, включая следующие переменные: исходный уровень HbA1c, возраст, пол, противодиабетические препараты при рандомизации (инсулин±метформин).
Пошаговая иерархия использовалась для контроля частоты ошибок типа I по ключевой эффективности конечных точек в следующем порядке: HbA1c, ГПН, МТ, HbA1c<7,0% (53 ммоль/ моль), САД и ДАД. Для каждой конечной точки сначала тестировали дозу 15 мг по сравнению с плацебо, а затем дозу 5 мг по сравнению с плацебо, если статистически значимый результат был достигнут для дозы 15 мг.
В анализах безопасности для НЯ 1-го и 2-го уровней (за исключением гипогликемии) были рассчитаны доверительные интервалы (ДИ) 95% для разницы рисков и р-значения (без поправки на множественность). Частота всех НЯ и НЯ, приведших к прекращению приема исследуемого препарата, также были обобщены [1].
Результаты
Из 8246 пациентов, рандомизированных в группу VERTIS CV, 1065 с СД2 и АСССЗ были включены в дополнительное исследование. Всего 18-недельный период наблюдения завершили 979 (91,9%) пациентов. Доля пациентов, завершивших прием исследуемого препарата до 18-й недели, была одинаковой в группах лечения (плацебо — 8,9%, эртуглифлозин 5 мг - 6,3%, эртуглифлозин 15 мг - 8,9%). Отказ пациента был самой частой причиной прекращения приема исследуемого препарата. Исходные демографические данные и характеристики были схожими во всех группах лечения (табл. 1).
В целом 68,2% пациентов были мужчинами со средним возрастом (SD) 64,8±7,8 года и средней продолжительностью СД2 16,7±9,0 лет. Исходно среднее значение HbA1c составляло 8,4±1,0% (68,4±10,4 ммоль/моль), ГПН составляла 172,3±57,0 мг/дл (9,6±3,2 ммоль/л), рСКФ - 73,7±20,4 мл/мин/1,73 м2. В целом 40,6% пациентов получали только инсулин, 59,4% получали инсулин и метформин. Большинство (75,7%) больных получали инсулин в качестве базально-болюсной терапии (отдельный инсулин промежуточного/длительного действия и инсулин короткого действия или предварительно смешанные инсулины промежуточного/длительного и короткого действия). Медиана (межквартильный диапазон) дозы инсулина на исходном уровне составил 58,0 (40-86) ЕД/сут (среднее - СО 70,3±45,1 ЕД/сут). Для пациентов, принимавших метформин в начале исследования, средняя доза метформина составила 2000 (15004050) мг/сут [1].
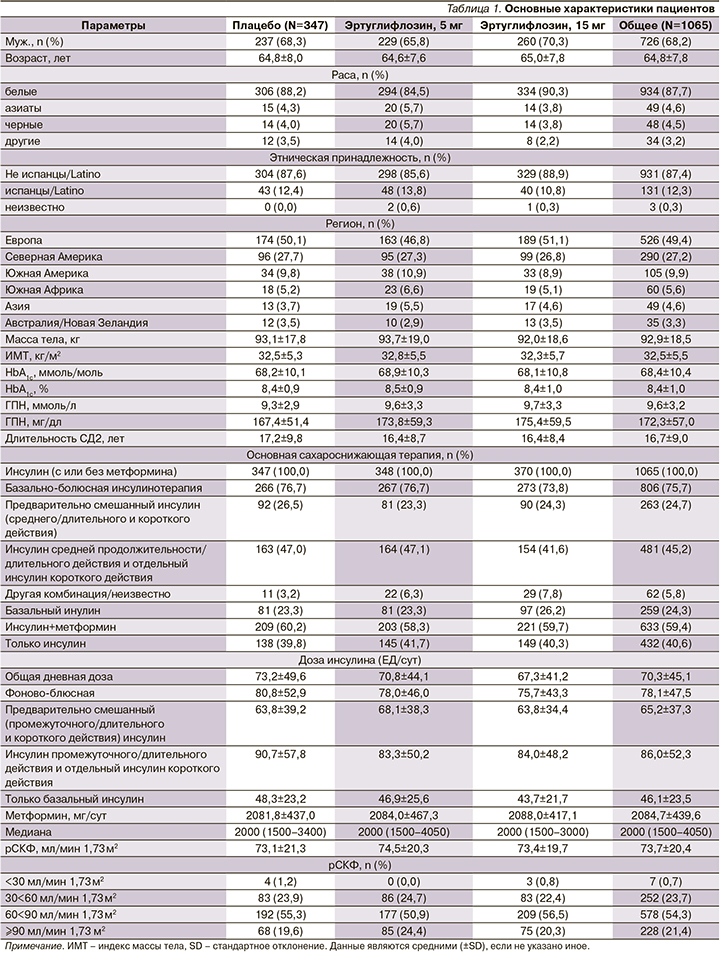
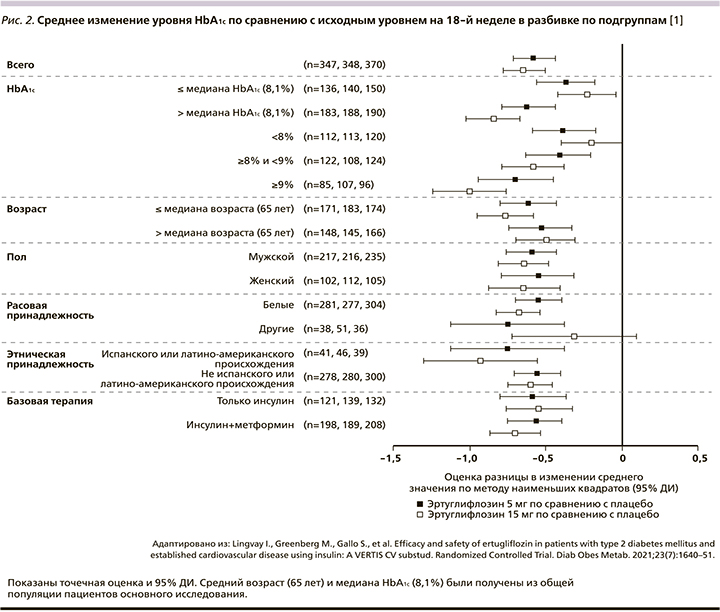
Эртуглифлозин 5 и 15 мг значительно снижал уровень HbA1c на 18-й неделе по сравнению с плацебо: значение LS с поправкой на плацебо 95% ДИ изменение: -0,58% (-0,71--0,44) и -0,65% (-0,78--0,51) соответственно; р<0,001 для обоих сравнений (рис. 1). Снижение было выше при применении эртуглифлозина по сравнению с плацебо во всех категориях подгрупп, включая пациентов с фоновым метформином или без него (рис. 2). Большинство пациентов, получивших эртуглифлозин 5 мг (20,7%) и 15 мг (21,1%), по сравнению с плацебо (10,7%) имели HbA1c<7,0% (53 ммоль/ моль) на 18-й неделе (табл. 2).
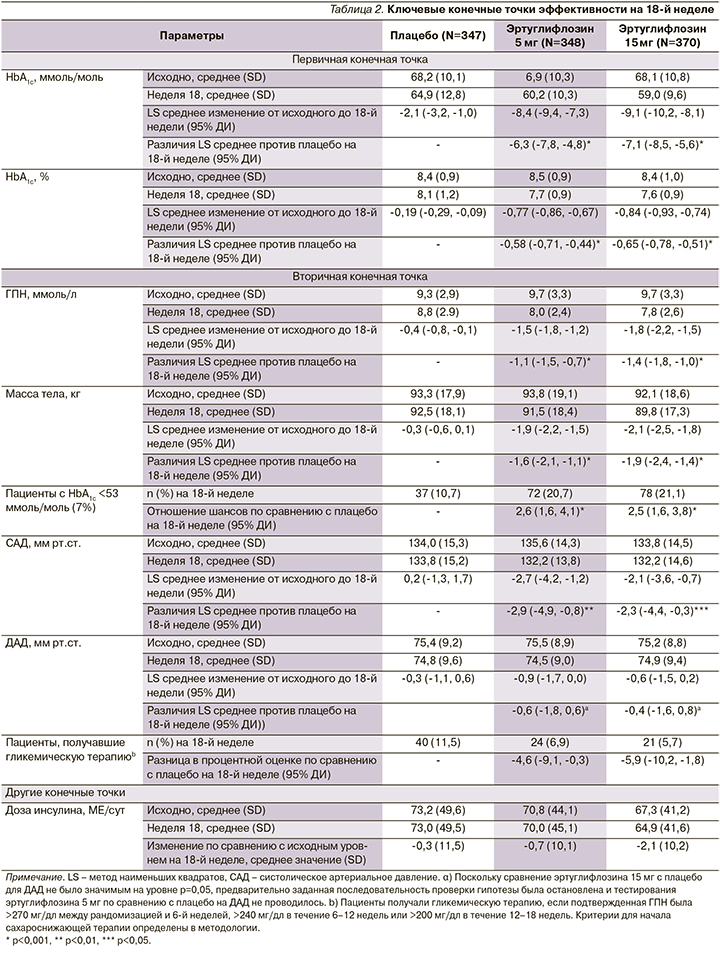
Основанные на модели шансы наличия HbA1c<7,0% (53 ммоль/моль) на 18-й неделе были выше при приеме 5 и 15 мг эртуглифлозина по сравнению с плацебо (табл. 2; р<0,001 для обоих сравнений). Значительно большее снижение ГПН по сравнению с исходным уровнем на 18-й неделе было отмечено для обеих доз эртуглифлозина (табл. 2, рис. 2В) по сравнению с плацебо. К 18-й неделе у пациентов, получавших гипогликемическую терапию, ГПН была ниже при применении эртуглифлозина 5 (6,9%) и 15 мг (5,7%) по сравнению с плацебо (11,5%). На 18-й неделе произошло небольшое снижение средней (SD) суточной дозы инсулина в группе эртуглифлозина в дозе 15 мг по сравнению с плацебо (табл. 2) [1].
Обе дозы эртуглифлозина обеспечили значительно большее снижение МТ по сравнению с исходным уровнем (табл. 2, рис. 2C) на 18-й неделе по сравнению с плацебо: среднее значение LS с поправкой на плацебо (95% ДИ изменение -1,6 [-2,1, -1,1] и -1,9 [-2,4, -1,4] кг соответственно; р<0,001 для обоих сравнений) [1].
Эртуглифлозин в дозе 5 и 15 мг обеспечивал значительно большее снижение САД, чем плацебо, по сравнению с исходным уровнем (табл. 2, рис. 2D). Изменение среднего значения LS с поправкой на плацебо (95% ДИ: -2,9 [-4,9, -0,8] и -2,3 [-4,4, -0,3] мм рт.ст. соответственно; р<0,01 и 0,05 соответственно).
Среднее снижение ДАД с поправкой на плацебо (95% ДИ) по сравнению с исходным уровнем на 18-й неделе (табл. 2, рис. 2Е) составило -0,6 мм рт.ст. (-1,8, 0,6) для 5 мг эртуглифлозина и -0,4 мм рт.ст. (-1,6, 0,8) для эртуглифлозина 15 мг (табл. 2; р>0,05 для обоих) [1].
Общая частота НЯ и серьезных НЯ (СНЯ) была одинаковой в группах лечения (табл. 3).

Для всех НЯ табл. 3 содержит события, которые произошли между первой дозой лечения и 14 днями после последней дозы лечения, за исключением смерти, о которой сообщается за весь период исследования независимо от того, произошло ли НЯ между первой дозой лечения и 14 днями после последней дозы лечения или с началом НЯ более чем через 14 дней после приема последней дозы исследуемого препарата.
a) Из 11 смертей в субисследовании 8 были расценены как смерть от сердечно-сосудистых заболеваний (включая 1 пациента в группе плацебо), 1 был связан с метастатическим поражением рака желудка, 1 з-за инсульта, а оставшаяся смерть наступила по неизвестной причине.
b) N=237 для плацебо, 229 для эртуглифлозина 5 мг и 260 для эртуглифлозина 15 мг.
c) N=110 для плацебо, 119 для эртуглифлозина 5 мг и 110 для эртуглифлозина 15 мг.
d) Симптоматическая гипогликемия определялась как событие с клиническими симптомами, описанными исследователем как гипогликемия (биохимическая документация не проводилась).
e) Документально подтвержденная гипогликемия определялась как эпизод с уровнем глюкозы <70 мг/ дл (<3,9 ммоль/л) с симптомами или без них.
f) Были подсчитаны все применимые эпизоды, включая множественные эпизоды у одного и того же пациента.
g) Тяжелая гипогликемия определялась как эпизод симптоматической гипогликемии, требующий медицинской или немедицинской помощи, независимо от того, была ли получена такая помощь, и независимо от биохимической документации
Частота НЯ, приводивших к прекращению приема исследуемого препарата, была низкой (<4% случаев) среди пациентов любой группы. Во время дополнительного исследования было 11 смертей (плацебо - 1, эртуглифлозин 5 мг — 4, эртуглифлозин 15 мг — 6; табл. 3). У 4 пациентов были фатальные НЯ, которые произошли более чем через 14 дней после приема последней дозы исследуемого препарата (эртуглифлозин 5 мг — 1, эртуглифлозин 15 мг — 3) [1].
У женщин частота ГМИ была выше при приеме 5 мг эртуглифлозина (3,4%; р=0,05) и эртуглифлозина 15 мг (3,6%; р=0,04) по сравнению с плацебо (0,0%) (табл. 3). У мужчин были небольшие различия в частоте развития ГМИ при применении эртуглифлозина 5 мг (1,7%) и 15 мг (2,7%) по сравнению с плацебо (0,8%). Не было пациентов, прекративших прием исследуемого препарата из-за СНЯ или ГМИ.
Частота НЯ ИМП была сходной во всех группах лечения (см. табл. 3). У двух пациентов были ИМП (эртуглифлозин 15 мг — 1, плацебо — 1), включая одно СНЯ (плацебо — 1) — ИМП, которое привело к прекращению приема исследуемого медикамента.
Частота симптоматической, документально подтвержденной, и тяжелой гипогликемии была одинаковой по группам лечения. Большее число эпизодов задокументированной и тяжелой гипогликемии наблюдалось при применении эртуглифлозина по сравнению с плацебо (см. табл. 3). Частота гиповолемии была низкой (<2,5%) и сходной на протяжении всего лечения. Было 5 пациентов с СНЯ — гиповолемией (эртуглифлозин 5 мг — 1, эртуглифлозин 15 мг — 1, плацебо — 3) и один с НЯ в виде гиповолемии (эртуглифлозин 15 мг), что привело к прекращению приема исследуемого препарата [1].
Обсуждение
Дополнительное исследование VERTIS CV продемонстрировало, что эртуглифлозин обеспечивал клинически значимое по сравнению с плацебо снижение уровня HbA1c и ГПН на 18-й неделе у пациентов с СД2 и АСССЗ, получавших инсулин >20 ЕД/ сут. Кроме того, большинство пациентов, принимавших эртуглифлозин, соответствовали целевому показателю HbA1c<7,0% (53 ммоль/моль). Среди других преимуществ эртуглифлозина по сравнению с плацебо отмечено умеренное снижение МТ и САД [1].
Ингибиторы SGLT2 привлекательны для использования в сочетании с экзогенным инсулином, поскольку два класса препаратов имеют взаимодополняющие механизмы гипогликемического действия. Инсулин способствует клеточному поглощению глюкозы в периферических тканях, особенно в жировой и мышечной, и снижает глюконеогенез в печени [21], тогда как ингибиторы SGLT2 уменьшают реабсорбцию глюкозы, выделяемую почками [22]. Кроме того, ингибиторы SGLT2 уравновешивают нежелательный эффект увеличения массы тела при инсулинотерапии.
Эффективность и безопасность эртуглифлозина, представленные здесь, аналогичны таковым в исследованиях других SGLT2. В дополнительном оценочном исследовании CANagliflozin Cardiovascular (CANVAS - основное исследование) канаглифлозин добавляли к инсулинотерапии (>20 МЕ/ сут) пациентам с СД2 и преобладанием АСССЗ или при повышенном риске АСССЗ. В этом исследовании выявлено улучшение гликемического контроля и снижение МТ и САД через 18 недель [23]. Гипогликемия наблюдалась в группах канаглифлозина по сравнению с плацебо.
В другом исследовании пациентов с СД2 эмпаглифлозин добавляли к многократным ежедневным инъекциям инсулина (базального или прандиального±метформин) или к базальному инсулину (>20 МЕ/сут± метформин, и/или производные сульфонилмочевины). Эмпаглифлозин улучшал гликемический контроль и снижал МТ с такой же частотой гипогликемии, как и у плацебо через 18 недель (об эпизодах гипогликемии не сообщалось) [24, 25].
В исследовании пациентов с СД2 добавление дапаглифлозина к инсулину (>30 МЕ/сут±метформин±другое пероральное средство) улучшало гликемический контроль, стабилизировало дозирование инсулина и снижало МТ с более высокой частотой эпизодов гипогликемии по сравнению с плацебо через 24 недели. О снижении суточной дозы инсулина сообщалось в этом субисследовании и в других исследованиях, в которых оценивались ингибиторы SGLT2, несмотря на различия в дизайне, временных точках и дозах [23-25, 27].
Эртуглифлозин (5 и 15 мг) в целом хорошо переносился пациентами с СД2 и АСССЗ, которые получали инсулин, а профиль безопасности соответствовал таковому у других ингибиторов SGLT2 [28].
Общая частота НЯ, СНЯ и прекращение приема из-за НЯ были одинаковыми во всех группах. Большинство смертей были расценены как сердечно-сосудистые. Кардиоваскулярная безопасность оценивалась как часть общего исследования VERTIS CV [14].
Частота ГМИ была выше у женщин, принимавших эртуглифлозин, по сравнению с плацебо, но общий процент заболевших пациентов был низким. Не было случаев прекращения приема исследуемого препарата из-за ГМИ. Возникновение ИМП и гиповолемия также были редкими и одинаковыми в группах лечения. Хотя число эпизодов документированной и тяжелой гипогликемии было численно выше в группах с эртуглифлозином, общая частота симптоматической и документально подтвержденной гипогликемии была одинаковой во всех группах лечения. В группах эртуглифлозина по сравнению с плацебо наблюдалось большее снижение дозы инсулина; как и в случае, когда добавление к инсулину сахароснижающих препаратов других классов уменьшает потребность в инсулине и снижает риск гипогликемии [29, 30].
Результаты этого дополнительного исследования могут иметь клиническое значение для популяции, которой обычно трудно управлять в клинической практике (т.е. у пациентов с длительно текущим СД2, установленным АССЗ и неадекватно контролируемой инсулинотерапией) с точки зрения безопасности и простоты в достижении целей гликемического контроля.
Преимущества улучшенного гликемического контроля и снижение МТ и САД, наблюдаемые при применении эртуглифлозина, вместе с возможным снижением риска госпитализации по поводу сердечной недостаточности [14, 15] и почечных исходов [14, 16] наблюдается при лечении эртуглифлозином по сравнению с плацебо в общем исследовании VERTIS CV [14] и может изменить течение и улучшить прогноз заболевания в этой важной популяции.
У настоящего исследования есть ряд потенциальных ограничений, в т.ч. его относительно короткая продолжительность и характеристики изучаемой популяции, ограниченная пациентами с СД2 и распространенным АСССЗ. Однако поскольку 18-недельной продолжительности было достаточно, чтобы наблюдать плато ответа HbA1c [1], результаты могут помочь клиницистам в установлении целей гликемического контроля над пациентами с СД2, начинающих терапию эртуглифлозином на фоне инсулина.
Протокол дополнительного исследования требовал, чтобы пациенты поддерживали стабильную дозу инсулина в течение 18 недель, что не обязательно соответствует клинической практике, когда дозы инсулина могут быть увеличены для улучшения гликемического контроля. Тем не менее, такой дизайн исследования позволил оценить гликемические эффекты эртуглифлозина [1]. Оценка долгосрочной гликемической эффективности была нарушена из-за изменений дозы инсулина после 18-й недели по усмотрению исследователя в соответствии с применимыми местными правилами. В других плацебо-контролируемых исследованиях ингибиторов SGLT2, добавленных к инсулину, гликемический контроль наблюдался при более ранних временных точках (18—24 недели) и в целом аналогичным тому, который был достигнут при длительном лечении за период (48-104 недели). Однако выводы об эффекте длительного гликемического контроля в этих исследованиях можно сделать только с учетом изменения в дизайне в конце плацебо-контролируемого периода [23, 24, 27]. Кроме того, это дополнительное исследование не оценивало долгосрочной безопасности измененного режима, дозировок и добавление новых препаратов сахароснижающей терапии за пределами плацебо-контролируемого периода, что может искажать результаты, особенно в отношении оценки гипогликемии. Несмотря на то что это подисследование включало только подгруппу пациентов, получавших инсулин >20 ЕД/сут, число пациентов и размер выборки в исследовании VERTIS CV были больше, чем в аналогичных исследованиях, и это обеспечило достаточную мощность для оценки конечных точек [24, 26].
Хотя это дополнительное исследование проводилось исключительно в отношении пациентов с СД2 и АСССЗ, предыдущие исследования с эртуглифлозином продемонстрировали его безопасность и гликемическую эффективность в виде монотерапии или в комбинации с метформином, и/или другими сахароснижающими препаратами в качестве терапии второй или третьей линии [31-37]. Таким образом, результаты, вероятно, будут более широко применимы к пациентам с СД2 и без АСССЗ.
Заключение
Эртуглифлозин в комбинации с инсулином (>20 ЕД/сут) обеспечивает пациентам с СД2 и АСССЗ клинически значимое улучшение гликемического контроля, а также преимущества в МТ и САД по сравнению с плацебо. Эртуглифлозин в целом хорошо переносится.
Наиболее частыми НЯ, связанными с лечением, являются ГМИ у женщин.
Эртуглифлозин может быть полезным вариантом лечения пациентов с СД2, получающих инсулин, которым нужен дополнительный гликемический контроль.



